 Studies presented at the ASCO Annual Meeting in the field of noncolorectal gastrointestinal cancer both reaffirmed certain standards of care and introduced some practice-changing data, according to A. Craig Lockhart, MD, of Washington University in St. Louis.
Studies presented at the ASCO Annual Meeting in the field of noncolorectal gastrointestinal cancer both reaffirmed certain standards of care and introduced some practice-changing data, according to A. Craig Lockhart, MD, of Washington University in St. Louis.
Perioperative Therapy for Gastric Cancer
After curative resection of gastric or gastroesophageal junction cancer, the combination of ECF (epirubicin, cisplatin, and infusional 5-FU) is not more efficacious or safer than the standard of bolus fluorouracil (5-FU) and leucovorin when incorporated into a chemoradiation scheme, according to findings of the Cancer and Leukemia Group B (CALGB) 80101 trial.1
Among 546 patients with resected tumors, each regimen was given before and after chemoradiation. The postchemoradiation doses of epirubicin and cisplatin in ECF were reduced because of toxicity concerns. Trial results showed that the two groups did not differ significantly regarding disease-free and overall survival, and had similar rates of toxicities.
“A question that came up is, did the downgrading of the epirubicin and cisplatin doses potentially impact the efficacy [of the ECF]. I don’t think we have the answer at this time,” Dr. Lockhart said.
“It does not appear that ECF is any better than the standard Intergroup 0116 approach” of 5-FU/leucovorin, he commented. “Incorporating ECF, making the 0116 regimen more complicated by adding other drugs to it, does not appear to improve survival, so the 0116 approach is still a standard.”
Adjuvant XELOX in Gastric Cancer
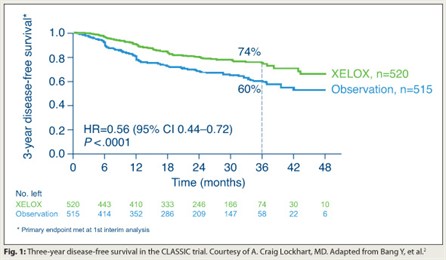 The phase III CLASSIC trial showed that giving XELOX (capecitabine [Xeloda] plus oxaliplatin) in the adjuvant setting improves disease-free survival in gastric cancer even after the more extensive D2 resection.2
The phase III CLASSIC trial showed that giving XELOX (capecitabine [Xeloda] plus oxaliplatin) in the adjuvant setting improves disease-free survival in gastric cancer even after the more extensive D2 resection.2
In all, 1,035 patients in South Korea, China, and Taiwan underwent a D2 resection and were randomly assigned to XELOX or observation. Median dose intensity was 85% for capecitabine and 98% for oxaliplatin, and there were no unexpected toxicities.
The 3-year rate of disease-free survival was better with XELOX (74% vs 60%; HR = 0.56; P < .0001; Fig. 1). Rates of recurrence were reduced at all sites: locoregional, peritoneal, and distant. Overall survival did not differ. “But this study had not been going on long enough to look at that definitively,” Dr. Lockhart noted.
“Adjuvant XELOX is a standard option following a D2 resection,” he concluded. “In my opinion, this is practice-changing.”
Second-line Chemotherapy for Advanced Gastric Cancer
Second-line chemotherapy has a 1.3-month survival benefit in fit patients with advanced gastric cancer, a phase III trial revealed.3
The 193 patients were randomly assigned 2:1 to second-line chemotherapy (docetaxel or irinotecan) or best supportive care. Relative dose intensity was 95% for docetaxel and 93% for irinotecan.
With a median follow-up of 17 months, overall survival was better with second-line chemotherapy than with best supportive care (5.1 vs 3.8 months, P = .009). Benefit did not differ between docetaxel and irinotecan recipients.
“Interestingly, no quality-of-life data were presented,” Dr. Lockhart noted. However, there was a trend toward a lower rate of grade 3/4 fatigue with chemotherapy vs best supportive care (18% vs 27%). And a similar study of second-line irinotecan found an improvement in symptoms.4
“Second-line chemotherapy can be considered a standard approach for patients with good performance status…who want to receive second-line therapy,” he maintained.
Sorafenib for Child-Pugh B Patients with Hepatocellular Carcinoma
 Patients with advanced hepatocellular carcinoma having Child-Pugh B class liver function may derive some benefit from sorafenib (Nexavar) and can start on the full dose, according to new data.5
Patients with advanced hepatocellular carcinoma having Child-Pugh B class liver function may derive some benefit from sorafenib (Nexavar) and can start on the full dose, according to new data.5
In a second interim analysis of the GIDEON study, which is assessing sorafenib therapy in routine clinical practice, investigators analyzed data from 1,612 patients. The Child-Pugh class was A, B, and C in 70%, 27%, and 3% of patients, respectively.
The majority of patients received the full 800-mg dose of sorafenib, regardless of Child-Pugh class. However, the median treatment duration fell off with class—it was 14, 9, and 4 weeks with A, B, and C class. The corresponding rate of adverse events leading to treatment discontinuation was 24%, 38%, and 51%.
Median overall survival was 10.3, 4.8, and 2.0 months. The 10.3 months in patients with class A liver function is “reassuring” because it mirrors that in clinical trials, according to Dr. Lockhart. The results suggest the same dosing strategy can be used for patients with Child-Pugh A and B class, he said.
“Right now, there is no indication to treat Child-Pugh C patients with sorafenib,” Dr. Lockhart maintained. “I would argue that Child-Pugh B patients probably don’t benefit much from sorafenib either, but if you don’t have other options, such as a clinical trial, I don’t think it’s wrong to [offer it].”
Cisplatin in Chemoradiation for Anal Cancer
Cisplatin is not superior to the standard mitomycin when used with 5-FU in chemoradiation for rectal cancer, according to long-term results of the Radiation Therapy Oncology Group (RTOG) 98-11 trial.6
A total of 650 patients were randomly assigned to cisplatin (induction with 5-FU and cisplatin before chemoradiation with 5-FU and cisplatin) or mitomycin (only chemoradiation with 5-FU and mitomycin). Compared with cisplatin, mitomycin yielded higher 5-year rates of disease-free survival (67.7% vs 57.6%, P = .004) and overall survival (78.2% vs 70.5%, P = .02), and a trend toward a lower rate of colostomy failure (11.9% vs 17.3%, P = .075). Male sex, a primary tumor larger than 5 cm, and clinically positive lymph nodes were all independent adverse prognostic factors.
“The results remain somewhat surprising as we thought cisplatin was going to potentially change the way that we practice. It did not,” Dr. Lockhart asserted. “5-FU/mitomycin along with radiation therapy remains the standard for patients with anal canal carcinomas.” ■
Disclosure: Dr. Lockhart has received research funding from Allos, Amgen, Bayer, Imclone/Lilly, Novartis, Merck, Millennium, Pfizer, Sanofi-Aventis, and Zenyaku.
SIDEBAR: Will East-West Differences Limit Transferability of Clinical Trial Results?
References
1. Fuchs CS, Tepper JE, Niedzwiecki D, et al: Intergroup trial CALGB 80101. 2011 ASCO Annual Meeting. Abstract 4003. Presented June 7, 2011.
2. Bang Y, Kim YW, Yang H, et al: Adjuvant capecitabine and oxaliplatin for gastric cancer: Results of the phase III CLASSIC trial. 2011 ASCO Annual Meeting. Abstract LBA4002. Presented June 7, 2011.
3. Park SH, Lim DH, Park K, et al: A multicenter, randomized phase III trial comparing second-line chemotherapy plus best supportive care with BSC alone for pretreated advanced gastric cancer. 2011 ASCO Annual Meeting. Abstract 4004. Presented June 7, 2011.
4. Thuss-Patience PC, Kretzschmar A, Bichev D, et al: Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: A randomised phase III study of the AIO. Eur J Cancer July 8, 2011 (early release online).
5. Marrero JA, Lencioni R, Kudo M, et al: Global Investigation of Therapeutic Decisions in Hepatocellular Carcinoma and of its Treatment with Sorafenib (GIDEON) second interim analysis in more than 1,500 patients. 2011 ASCO Annual Meeting. Abstract 4001. Presented June 7, 2011.
6. Gunderson LL, Winter KA, Ajani JA, et al: Long-term update of U.S. GI intergroup RTOG 98-11 phase III trial for anal carcinoma. 2011 ASCO Annual Meeting. Abstract 4005. Presented June 7, 2011.

