An updated overall survival analysis of the phase III HIMALAYA study, now at 5 years, confirms the benefit for the STRIDE regimen of the monoclonal antibodies durvalumab plus tremelimumab in unresectable hepatocellular carcinoma.1 [The STRIDE regimen consists of a single priming dose of tremelimumab followed by a regular-interval dose of durvalumab.] At the European Society for Medical Oncology (ESMO) Congress 2024, investigators reported an unprecedented doubling of 5-year survival, which was 19.6% with STRIDE vs 9.4% with sorafenib alone, a 24% reduction in the risk of death. This benefit was accentuated in patients achieving disease control, and no additional safety concerns were observed with extended follow-up.1

Lorenza Rimassa, MD
“These results set a new benchmark, with one in five patients alive with STRIDE at 5 years,” said Lorenza Rimassa, MD, Associate Professor of Medical Oncology in the Department of Biomedical Sciences, Humanitas University in Pieve Emanuele and Humanitas Cancer Center, IRCCS Humanitas Research Hospital in Rozzano (Milan), Italy. “Another important finding is that we observed it is not necessary to long-term survival to have a conventional objective response by RECIST v.1 criteria,” she added. The improvement in survival across multiple tumor response categories provides novel insights on the clinical benefit of dual immune checkpoint inhibition beyond conventional measures of response, she said.
Now, with 5 years of extensive analyses, Dr. Rimassa shared these comments: “The data are mature…. We can be comfortable with it in our clinical practices and in sharing it with our patients.”
About HIMALAYA
The phase III study enrolled 1,171 patients and randomly assigned them to one of three regimens: STRIDE (single dose of tremelimumab at 300 mg plus monthly dose of durvalumab at 1,500 mg followed by durvalumab at 1,500 mg every 4 weeks); sorafenib at 400 mg twice daily; or single-agent durvalumab at 1,500 mg every 4 weeks.
A survival benefit was reported in previous analyses, with durability of survival observed at 48 months.2,3 At the ESMO Congress, Dr. Rimassa reported the first 5-year overall survival analysis and survival according to tumor response measures after a median follow-up of about 63 months (Table 1).
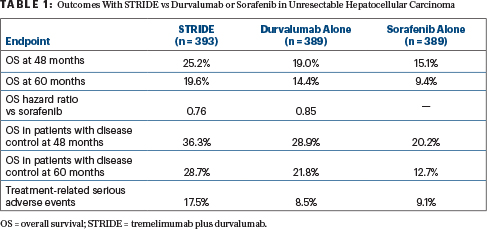
Those patients achieving disease control had an overall survival rate of 28.7% with the STRIDE regimen vs 12.7% with sorafenib. In addition, an exploratory analysis of the depth of response showed that more patients treated with the STRIDE regimen experienced deep responses leading to longer survival compared with sorafenib:
- Depth of response > 75% at 60 months: 72.7% vs 33.3%
- Depth of response > 50% and ≤ 75%: 57.8% vs 32.1%.
“Even small reductions in tumor diameters can be associated with long-term survival, though, of course, patients with deeper responses are more represented among long-term survivors,” she indicated.

Ghassan K. Abou-Alfa, MD, MBA, FASCO
Dr. Rimassa was reassured that the rate of treatment-related serious adverse events with STRIDE did not change from the primary analysis. HIMALAYA global lead and principal investigator Ghassan K. Abou-Alfa, MD, MBA, FASCO, commented on this observation: “The safety profile didn’t change at 5 years. It’s incredible that we are able to see people function with limited adverse events…. Patients can keep going on with their lives, coming in for one injection once a month, and that’s it.”
DISCLOSURE: Dr. Rimassa has received honoraria (lecture fees) from AstraZeneca, Bayer, BMS Guerbet, Incyte, Ipsen, Roche, and Servier; has served as a consultant or advisor to AbbVie, AstraZeneca, Basilea, Bayer, BMS, Elevar Therapeutics, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Jazz Pharmaceuticals, MSD, Nerviano Medial Sciences, Roche, Servier, Taiho Oncology, and Zymeworks; has received institutional research funding from Agios, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Servier, Taiho Oncology, TransThera Sciences, and Zymeworks; and has been reimbursed for travel and accommodations for congresses from AstraZeneca. Dr. Abou-Alfa has served as a consultant or advisor to Eisai, Ipsen, AstraZeneca, Yiviva, Roche/Genentech, Autem, Exelixis, QED Therapeutics, Berry Genomics, Vector Health, Servier, J-Pharma, and AbbVie.
EXPERT POINT OF VIEW

James J. Harding, MD
“At the present time, there are multiple standard-of-care options for front-line treatment of patients with advanced hepatocellular carcinoma,” stated James J. Harding, MD, a gastrointestinal medical oncologist and early drug development specialist at Memorial Sloan Kettering Cancer Center, New York. “The general consensus, based on the available data, is that combination anti–PD-1–based therapy is life-prolonging in this space. There are multiple ways you can get there, but the two regimens that are really critical here are either atezolizumab plus bevacizumab or tremelimumab plus durvalumab.” He added that the combination of tyrosine kinase inhibitors with either PD-1 or PD-L1 antibodies is another approach being studied.
Dual blockade of PD-1 and CTLA-4, as is achieved with durvalumab and tremelimu-mab, is based on extensive preclinical data in a number of tumor types. In melanoma, overall survival is being observed 10 years out with ipilimumab and nivolumab. In hepatocellular carcinoma, “this is a well-vetted approach” and is supported by “robust” phase II data, he said.
Focusing on the phase III HIMALAYA trial, Dr. Harding shared these comments: “The impressive aspect of the data is that we see a continued overall benefit at 5 years—estimated 20% alive vs 9% with dual treatment vs tyrosine kinase-based therapy. This is reminiscent of what we’ve seen in melanoma and kidney cancer with dual blockade of CTLA-4 and PD-1…. The modest response rate of 20.1%, vs 5%, is generally consistent with the concept that objective response is not a surrogate for overall survival. We see that whether or not patients responded, there are still patients at the tail of the curve. Certainly, there is an enrichment in those who attain a response or have a high degree of tumor shrinkage, but we can see that some patients who received this therapy didn’t receive anything else.”
Dr. Harding concluded: “This begs the question, are we actually curing a small patient subset, and how can we improve on this regimen? We all have patients from years ago who are still in our clinics…. The follow-up data here for HIMALAYA support this regimen [STRIDE] as a life-prolonging therapy for patients with advanced hepatocellular carcinoma.”
DISCLOSURE: Dr. Harding has received research support from NCI P30-CA008748, NCI U01 CA238444 04, the Society of Memorial Sloan Kettering Cancer Center, Experimental Therapeutics Center, and Cycle for Survival as well as additional research support from AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, CytomX, Debiopharm, Eli Lilly, Genoscience, Incyte, Kinnate Biopharma, Loxo @ Lilly, Novartis, Polaris, Pfizer, Tvardi, Zymeworks, and Yiviva; and has received consulting fees from Adaptimmune, AstraZeneca, Bristol Myers Squibb, Exelixis, Elevar, Eisai, Genoscience (uncompensated), Hepion, Imvax, Merck (data and safety monitoring board), Medivir, QED, RayzeBio, Servier, Tempus, Tyra, and Zymeworks (uncompensated).
REFERENCES
1. Rimassa L, Chan SL, Sangro B, et al: Five-year overall survival (OS) and OS by tumor response measures from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. ESMO Congress 2024. Abstract 947MO. Presented September 16, 2024.
2. Abou-Alfa GK, Lau G, Kudo M, et al: Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid 1(8):EVIDoa2100070, 2022.
3. Sangro B, Chan SL, Kelley RK, et al: Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann Oncol 35:448-457, 2024.

