Late-breaking findings from the phase IIIb/IV, open-label DESTINY-Breast12 trial support the use of the antibody-drug conjugate fam-trastuzumab deruxtecan-nxki (T-DXd) in patients with HER2-positive metastatic breast cancer and brain metastases who have experienced disease progression on at least two therapies. The 12-month overall survival of patients both with and without brain metastases exceeded 90%, investigators reported at the European Society for Medical Oncology (ESMO) Congress 2024.1

Nancy U. Lin, MD
“T-DXd exhibited substantial and durable overall and intracranial clinical activity in a large patient cohort with HER2-positive metastatic breast cancer with stable and active brain metastases,” said Nancy U. Lin, MD, Associate Chief of Breast Oncology and Director of the Program for Patients with Breast Cancer Brain Metastases at Dana-Farber Cancer Institute, Boston. The data support the use of this agent, regardless of the status of brain metastases, she maintained.
The findings from DESTINY-Breast12 were preceded by “promising preliminary evidence” of intracranial activity with T-DXd in small prospective patient cohorts, retrospective studies, and exploratory analyses, she noted. In those studies, intracranial response rates have ranged from 44% to 73%. This led to DESTINY-Breast12, which is the largest prospective study of T-DXd in patients with stable or active brain metastases. The primary results were simultaneously published in Nature Medicine.2
About DESTINY-Breast12
Of 504 patients, 263 had brain metastases. Within this group, 157 had stable lesions, and 106 had active ones. Of the group with active brain metastases, 39 patients had not been previously treated, and 67 patients had received treatment or experienced disease progression after local treatment. Median follow-up of all patients was about 16 months.
“T-DXd showed consistent 12-month overall survival in patients with and without brain metastases,” Dr. Lin said. The 12-month survival rates were 90.6% for patients without baseline brain metastases and 90.3% for those with brain metastases. She focused the remainder of her presentation on patients with stable or active brain metastases.
Key Outcomes
As Dr. Lin reported, progression-free survival, progression-free survival in the central nervous system (CNS), overall response rate, and CNS response rate were all very promising, with outcomes shown in Table 1. In the post hoc analysis, median progression-free survival for patients with brain metastases was 17.3 months.
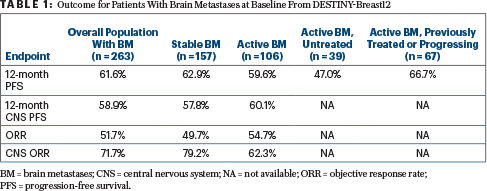
When the analysis was restricted to patients with active brain lesions that were measurable at baseline, the CNS response rate increased to 82.6% in previously untreated patients and was 50.0% in patients previously treated or experiencing disease progression, she reported.
Toxicity in Patients With Brain Metastases
The safety profile of T-DXd was consistent with previous reports. Grade ≥ 3 adverse events possibly related to treatment occurred in about half of the patients regardless of the presence of brain metastases, but those leading to discontinuation of therapy were observed in 15% of the cohort with brain metastases and 9% of those without brain metastases. Approximately 3% of each group died of a potentially treatment-related cause.
Interstitial lung disease and pneumonitis remain important safety risks with this agent, Dr. Lin emphasized. There were six deaths related to interstitial lung disease in the cohort with brain metastases, five of which were in patients taking steroids; none occurred in patients receiving prophylaxis for Pneumocystis pneumonia (PCP). In fact, four of the patients with interstitial lung disease had co-occurring opportunistic infections. There were three deaths related to interstitial lung disease among patients without brain metastases. “In patients with brain metastases on concomitant steroids, careful attention to PCP prophylaxis and workup for opportunistic infections is warranted,” Dr. Lin stated.
EXPERT POINT OF VIEW

Cristina Saura, MD, PhD
The invited discussant of DESTINY-Breast12 was Cristina Saura, MD, PhD, Head of the Breast Cancer Unit at Vall d’Hebron University Hospital, Barcelona. “Although DESTINY-Breast12 has no formal planned statistical hypothesis, it is relevant for two reasons,” she said. “First, it allowed 500 patients to receive fam-trastuzumab deruxtecan-nxki (T-DXd) under the indication studied in the DESTINY-Breast03 trial,3 which led to the approval of the drug in the second line and beyond for patients with HER2-positive metastatic breast cancer. The fact that this phase IIIB/IV trial was carried out not only provided access to the drug in countries where it was not yet reimbursed, but also enabled professionals to gain experience in managing the drug in a clinical trial setting,” Dr. Saura said. “Second, DESTINY-Breast12 included a population for whom there has been very limited evidence—patients with brain metastases and specifically for those patients with active brain metastases.”
The optimal systemic treatment of brain metastases has been limited by scant randomized trial evidence, primarily the data from the HER2CLIMB trial of tucatinib plus trastuzumab and capecitabine in the second-line and later setting.4 With that regimen, there was a statistically significant and clinically meaningful improvement in outcomes, with median progression-free survival of 7.8 months and median overall survival of 21.9 months with the triplet.
Now, more support has emerged for using T-DXd instead. Before -DESTINY-Breast12, Dr. Saura noted, evidence for using T-DXd in patients with active brain metastases, “while encouraging,” came from small, nonrandomized or retrospective studies. “With the data presented by Dr. Lin, we now have more evidence. The reported median progression-free survival of more than 17 months in the brain metastases cohort is very promising for this population of patients with historically poor prognosis.”
Of particular interest, she continued, are patients with active brain metastases, who are often symptomatic. In the active-metastases previously untreated subset, the central nervous system response was 82.6%. “This is particularly interesting from a clinical standpoint. It reinforces my preference for systemic treatments that will be highly effective, allowing me to delay radiotherapy until later stages,” she said.
Dr. Saura concluded: “The preferred treatment option for second-line treatment of patients with active brain metastases had been the tucatinib, trastuzumab, and capecitabine combination. Now, I believe the preferred option should be T-DXd, regardless of whether the patient has active brain metastasis or not,” with the HER2CLIMB regimen moved to the third line.
DISCLOSURE: Dr. Lin reported financial relationships with Seattle Genetics, Daiichi Sankyo, AstraZeneca, Olema Pharmaceuticals, Blueprint Medicines, Janssen, Artera, Stemline/Menarin, Eisa, UptoDate, Olema Pharmaceuticals, Zion Pharmaceuticals, Pfizer, and Genentech. Dr. Saura reported financial relationships with AstraZeneca, AX Consulting, Byondis, Daiichi Sankyo, Eisai, Exact Sciences, Exeter Pharma, F. Hoffmann–LaRoche, Lilly, MedTech, Merck Sharp & Dohme, Novartis, Pfizer, Phillips Pharma Group, Pierre Fabre, Pint Pharma, Puma Biotechnology, Seagen, and Zymeworks.
REFERENCES
1. Lin N, Ciruelos EM, Jerusalem G, et al: Trastuzumab deruxtecan in patients with HER2+ advanced/metastatic breast cancer with or without brain metastases. ESMO Congress 2024. Abstract LBA18. Presented September 13, 2024.
2. Harbeck N, Ciruelos E, Jerusalem G, et al: Trastuzumab deruxtecan in HER2-positive advanced breast cancer with or without brain metastases: A phase 3b/4 trial. Nat Med. September 13, 2024 (early release online).
3. Hurvitz SA, Hegg R, Chung WP, et al: Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 401:105-117, 2023.
4. Murthy RK, Loi S, Okines A, et al: Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 382:597-609, 2020.

