Concurrent chemoradiation therapy followed by durvalumab has become the standard of care in patients with unresectable, locally advanced non–small cell lung cancer (NSCLC), based on the PACIFIC trial.1 However, clinical trials have predetermined inclusion and exclusion criteria, and they evaluate selective patient populations. To evaluate the real-world benefit of durvalumab, researchers at the Mayo Clinic examined their patients receiving the drug after chemoradiation therapy for unresectable advanced NSCLC. They presented their findings at the International Association for the Study of Lung Cancer (IASLC) 2022 World Conference on Lung Cancer.2
Real-world data provide meaningful insights into extended patient populations, help uncover rare side effects, demonstrate outcomes of patients who experience disease progression after durvalumab treatment, and generate hypotheses to be tested in future trials, the researchers noted in their poster.

Joel Rivera Concepcion, MD
“Treatment with chemoradiation followed by 1 year of durvalumab consolidation was effective and well tolerated in a real-world population,” said Joel Rivera Concepcion, MD, an advanced medical oncology fellow at the Mayo Clinic, Rochester, who conducted the retrospective study of 190 patients treated at the Mayo Clinic for unresectable advanced NSCLC between 2018 and 2020.
“Progression-free survival, overall survival, and toxicity results at 12 months were comparable to those previously reported in the PACIFIC trial. Performance status and PD-L1 expression were the strongest predictors for outcomes,” he reported.
Mayo Clinic Study Details
This study assessed overall survival, progression-free survival, time from first disease progression to objective tumor progression on the next-line treatment or death from any cause (modified progression-free survival-2 [MPFS-2]), sites of disease progression, and toxicity.
For the 190 patients in the study, who were treated between 2018 and 2020 at all Mayo Clinic sites, the median age was 67, and 24 patients (13%) were classified as having a poor performance status. The median number of durvalumab cycles (given at 10 mg/kg every 2 weeks) was 12. Patients were followed for a median of 14.8 months.
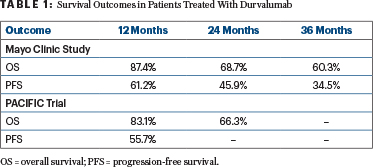
Overall survival and progression-free survival were similar to those reported in the PACIFIC trial (Table 1).
The most common distant metastatic disease sites at first disease progression were the brain (28%), bone (24%), and lungs (20%). Subsequent therapy for 66 patients whose disease progressed on durvalumab included radiation therapy in 24, chemotherapy in 33, immunotherapy in 7, targeted therapy in 3, and surgical resection in 1 patient.
Outcomes After Disease Progression
“In our study, we defined MPFS-2 differently. Instead of measuring the time from randomization to second disease progression or death (PFS-2), we calculated the rate from the first disease progression to the second disease progression or death (MPFS-2), Dr. Rivera Concepcion said.
In this study of 190 patients, there were 82 first disease progressions (66 disease progression and 16 deaths). Of the 66 first disease progressions, there were 36 second disease progressions, including 13 disease progressions and 23 deaths. This resulted in MPFS-2 rates of 32.1% at 1 year and 17.1% at 2 years (representing the percentage of patients who had not experienced disease progression or died), he reported.
Giving a more detailed look at the MPFS, the study showed that of the 66 patients with first disease progression, the progression-free survival rate at 1 year was 23% for patients with locoregional recurrence and 36% for those with distant metastasis. “This can be interpreted as locoregional recurrence showing worse outcomes than recurrence from distant metastasis,” Dr. Rivera Concepcion commented.
Patients completing all cycles of concurrent chemotherapy with radiation therapy, compared with those who did not, had no difference in disease progression.
“Although a small number of patients with ECOG performance status 2 were evaluated, the results showed a statistically significant increased risk in both disease progression (hazard ratio [HR] = 2.36, P = .01) and death (HR = 5.11, P = .001),” he said.
Expression of PD-L1 1% to 49% and expression of PD-L1 > 50%, when compared with PD-L1 0%, were associated with a statistically significant lower risk for death (P = .004 and P = .028, respectively).
The most common adverse event of any cause (all grades) was pneumonitis, which was seen in 29% of patients and was comparable to the rate in the PACIFIC trial. Common immune-related adverse events were hypothyroidism (12%), colitis (4%), and rash (3%).
DISCLOSURE: Dr. Rivera Concepcion reported no conflicts of interest.
REFERENCES
1. Antonia SJ, Villegas A, Daniel D, et al: Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med 377:1919-1929, 2017.
2. Rivera Concepcion J, Prodduturvar P, Gao RW, et al: Real world outcomes of durvalumab after chemoradiotherapy in unresectable advanced non-small cell lung cancer: The Mayo Clinic experience. 2022 World Conference on Lung Cancer. Abstract EP05.01-011. Presented August 6, 2022.

