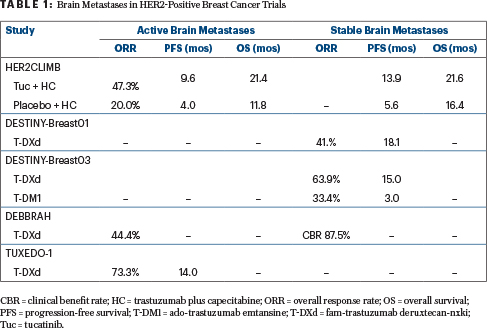
In the single-center phase II TUXEDO-1 trial of patients with advanced HER2-positive breast cancer, fam-trastuzumab-deruxtecan-nxki (T-DXd) showed efficacy in patients with active brain metastases, yielding intracranial responses in 73.3% of the population and a median progression-free survival of 14 months, according to Rupert Bartsch, MD, of the Medical University of Vienna, who presented the findings at the European Society for Medical Oncology (ESMO) Breast Cancer Congress 2022.1

Rupert Bartsch, MD
“The study reached its primary endpoint, with comparable intracranial and extracranial response rates observed, and suggested there is prolonged disease control by systemic treatment with T-DXd. Of note, quality-of-life neurocognitive functioning was maintained over the entire treatment period,” Dr. Bartsch said.
Background
Breast cancer is the second most common cause of brain metastases among solid tumors. Local therapy has long been the mainstay of treatment, but there is growing interest in systemic treatment to improve disease control.
To this end, several regimens have been used, including lapatinib plus capecitabine, neratinib plus capecitabine, and, more recently, tucatinib plus trastuzumab and capecitabine. This triplet yielded an intracranial response rate of 47% and a median progression-free survival of 9.5 months in patients with active lesions in the HER2CLIMB study,2 and it is regarded as the standard of care for this patient population.
There is less evidence regarding the activity of larger molecules, such as antibody-drug conjugates, but in the KAMILLA3 and DESTINY-Breast034 trials, ado-trastuzumab emtansine (T‑DM1) and T-DXd, respectively, were active in patients with stable brain metastases at baseline. Recently, the primary results of the DEBBRAH trial5 and hints from the TUXEDO-1 trial suggest that T-DXd has activity in patients with active brain metastases as well, said Dr. Bartsch.
Study Details
TUXEDO-1 included 15 patients with HER2-positive metastatic breast cancer and brain metastases that were either newly diagnosed or developed upon disease progression after local therapy. The brain metastases were untreated in 40% and for 60% had progressed after local therapy. Neurologic symptoms were present at baseline for 40% of patients. All patients were previously exposed to an anti-HER2–directed therapy, which for 60% included T-DM1. Patients received treatment with T-DXd at 5.4 mg/kg every 3 weeks until disease progression or unacceptable toxicity.
Key Findings and Safety Profile
The primary endpoint, objective response, was seen in 73.3% of the intent-to-treat population of 15 patients and 78.6% of the per-protocol population of 14 patients. The clinical benefit rate (≥ 6 months) was 86.7% and 92.9%, respectively. “Nearly all patients benefited to some degree from systemic treatment,” Dr. Bartsch noted.
In eight patients with measurable extracranial disease at baseline, the response rate was 62.5%. After a median follow-up of 11 months, the median progression-free survival was 14 months, and the median overall survival was not reached.
No new safety signals were observed. As for adverse events of special interest, one patient with preexisting diabetes experienced a grade 3 left-ventricular ejection fraction decrease, and one patient developed grade 2 interstitial lung disease, which completely resolved after 6 weeks of corticosteroid treatment. One patient died of grade 5 urosepsis unrelated to treatment.
Patients maintained global health status and physical, emotional, and cognitive function, after their decrease upon initiation of treatment. “Of note, in a trial conducted in patients with active brain metastases, cognitive functioning was maintained over the entire treatment period,” Dr. Bartsch said.
“Thus, TUXEDO-1 adds to the growing body of evidence that systemic treatment is feasible in patients with active brain metastasis. It more generally supports further investigation of antibody-drug conjugates in the context of secondary central nervous system malignancies,” Dr. Bartsch concluded.
DISCLOSURE: Dr. Bartsch reported financial relationships with AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Gilead, Grünenthal, MSD, Novartis, Pfizer, Pierre Fabre, Puma Biotechnology, Roche, and Seagen.
REFERENCES
1. Bartsch R, Berghoff AS, Furtner J, et al: Trastuzumab-deruxtecan in HER2-positive breast cancer patients with active brain metastases: Primary outcome analysis from the TUXEDO-1 trial. ESMO Breast Cancer Congress 2022. Abstract 165MO. Presented May 4, 2022.
2. Lin NU, Murthy RK, Abramson V, et al: Updated results of tucatinib vs placebo added to trastuzumab and capecitabine for patients with previously treated HER2-positive metastatic breast cancer with brain metastases (HER2CLIMB). 2021 San Antonio Breast Cancer Symposium. Abstract PD4-04. Presented December 8, 2021.
3. Montemurro F, Delaloge S, Barrios CH, et al: Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: Exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol 31:1350-1358, 2020.
4. Hurvitz S, Kim SB, Chung WP et al: Trastuzumab deruxtecan vs trastuzumab emtansine in patients with HER2+ metastatic breast cancer: Subgroup analyses from the randomized phase 3 study DESTINY-Breast03. 2021 San Antonio Breast Cancer Symposium. Abstract GS3-01. Presented December 9, 2021.
5. Vaz Batista M, Cortez P, Ruiz M, et al: Trastuzumab deruxtecan in patients with HER2[+] or HER2-low-expressing advanced breast cancer and central nervous system involvement: Preliminary results from the DEBBRAH phase 2 study. 2021 San Antonio Breast Cancer Symposium. Abstract PD4-06. Presented December 9, 2021.

