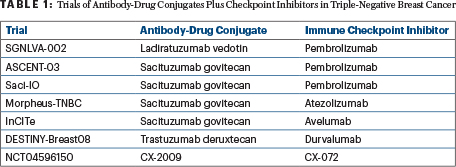
Preliminary results of the basket BEGONIA trial showed that the antibody-drug conjugate datopotamab deruxtecan (Dato-DXd), paired with the immune checkpoint inhibitor durvalumab, has strong activity in advanced or metastatic triple-negative breast cancer among patients not biomarker-selected for the trial. As a first-line treatment, the combination therapy produced responses in 74% of patients and exhibited a good safety profile, though mild stomatitis was common, as reported at the European Society for Medical Oncology (ESMO) Breast Cancer Congress 2022 by Peter Schmid, MD, PhD, of Barts Cancer Institute, Queen Mary University of London.1

Peter Schmid, MD, PhD
“Our preliminary results showed that the combination of Dato-DXd and durvalumab demonstrates a robust response rate in the first-line setting for metastatic/advanced triple-negative breast cancer. Responses were seen regardless of PD-L1 expression, which I think is an important finding of this trial,” Dr. Schmid said.
Combining immune checkpoint inhibitors with first-line chemotherapy has been shown to improve outcomes in patients with advanced or metastatic triple-negative breast cancer but only in patients expressing PD-L1, he pointed out.
KEY POINTS
- The basket BEGONIA trial is evaluating the immune checkpoint inhibitor durvalumab given in combination with novel agents, one being the TROP2-targeting antibody-drug conjugate datopotamab deruxtecan (Dato-DXd).
- Durvalumab plus Dato-DXd produced responses in 74% of patients.
- Patients were not enrolled based on PD-L1 status, and responses were seen regardless of PD-L1 expression.
- Mild stomatitis was seen in 69% of patients and was grade 3 in 14%.
Datopotamab deruxtecan is a TROP2-directed antibody-drug conjugate with a topoisomerase 1 inhibitor payload and tumor-selective, cleavable linker. This agent has shown antitumor activity as monotherapy in heavily pretreated patients with advanced triple-negative breast cancer. In the phase I TROPION-PanTumor01 study in heavily pretreated patients, responses to the single agent were observed in 34% of all patients and in 52% who had not previously received another a topoisomerase 1 inhibitor–based antibody-drug conjugate (eg, sacituzumab govitecan-hziy).2
These data are the first for Dato-DXd plus durvalumab in patients with newly diagnosed advanced or metastatic triple-negative breast cancer.
About BEGONIA
BEGONIA is an ongoing, two-part, open-label platform that is evaluating novel combinations of immunotherapies, including the checkpoint inhibitor durvalumab, as first-line therapy for advanced or metastatic triple-negative breast cancer. At the 2021 San Antonio Breast Cancer Symposium,3 Dr. Schmid reported a response rate of 66.7% for durvalumab paired with the antibody-drug conjugate fam-trastuzumab deruxtecan-nxki (T-DXd). In the control arm of durvalumab and paclitaxel, the response rate was 58.3%.
Dr. Schmid presented an analysis of Part 1 of BEGONIA, which included 30 patients randomly assigned to receive Dato-DXd plus durvalumab.4 Other randomizations are to combinations that include paclitaxel, capivasertib, oleclumab, or T-DXd, and that data are not presented here. Combinations that “graduate” from the first phase move into the expansion phase (phase II)—which is the case for Dato-DXd and for T-DXd at this point. In Part 2, an additional 27 patients will receive durvalumab plus Dato-DXd or durvalumab plus T-DXd. The primary endpoint of Part 2 will be objective response rate.
At the time of analysis, treatment with Dato-DXd plus durvalumab was ongoing for 24 patients (83%) from phase I. Visceral metastases were presented in 69% and lymph node metastases, in 76%. PD-L1 expression was low (< 5%) in 73% and high (≥ 5%) in 17%, and it was unknown or missing in 10%. Median follow-up was 3.9 months.
Outcomes and Safety Profile
Of 27 evaluable patients, 20 (74%) had confirmed objective responses, 2 of which (7%) were complete responses. Two additional patients demonstrated a reduction in tumor diameter but have not undergone a confirmation scan at this time; they are not included in the objective response rate. Responses were observed regardless of PD-L1 expression, Dr. Schmid reported.
The median time to response was 1.4 months. All responding patients were continuing to respond at data cutoff, and the median duration of response was not reached.
“We essentially saw a profile consistent with that of the single-agent activity for both agents,” Dr. Schmid noted. Treatment-related grade 3 adverse events occurred in 28%; 14% of patients required dose reductions of Dato-DXd, “but the majority could receive the dose as planned.” The most common side effects were stomatitis, alopecia, and low-grade nausea. Diarrhea was uncommon (14%, all grade 1), and no cases of interstitial lung disease or pneumonitis were observed.
The only grade 3 toxicity was stomatitis, which occurred in 14% and required dose reductions in about 14%. “We put new management guidelines in place, including prophylaxis, to reduce the incidence and severity of stomatitis,” he added.
Dato-DXd or T-DXd in Triple-Negative Disease?
Dr. Schmid has evaluated both Dato-DXd and T-DXd in triple-negative patients. A listener asked that if pressed, which would he choose: “It’s a question of which of my children do I like. It’s very difficult to answer,” he responded. “What I think is interesting is they are targeting two totally different targets. If you look at treatment sequencing, you can imagine that patients will get HER2-targeted therapy and a TROP2-targeted therapy sequentially. Which one to give first is something to work out.”
DISCLOSURE: Dr. Schmid has received research support from Astellas Pharma, AstraZeneca, F. Hoffmann–La Roche, Genentech, Medivation, Novartis, and OncoGenex Pharmaceuticals; has served as an advisor or consultant to AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, F. Hoffmann–La Roche, Merck, Novartis, Pfizer, and Puma Biotechnology; and has served on a data and safety monitoring board for Novartis.
REFERENCES
1. Schmid P, Jung KH, Wysocki PJ, et al: Datopotamab deruxtecan + durvalumab as first-line treatment for unresectable locally advanced/metastatic triple-negative breast cancer: Initial results from BEGONIA, a phase 1b/2 study. ESMO Breast Cancer Congress 2022. Abstract 166MO. Presented May 4, 2022.
2. Krop I, Juric D, Shimizu T, et al: Datopotamab deruxtecan in advanced/metastatic HER2-negative breast cancer: Results from the phase 1 TROPION-PanTumor01 study. 2021 San Antonio Breast Cancer Symposium. Abstract GS1-05. Presented December 7, 2021.
3. Schmid P, Nowecki Z, Im SA, et al: BEGONIA: Phase 1b/2 study of durvalumab combinations in locally advanced/metastatic triple-negative breast cancer: Results from Arm 1 durvalumab + paclitaxel, Arm 2 durvalumab + paclitaxel + capivasertib, and Arm 5 durvalumab + paclitaxel + oleclumab. 2021 San Antonio Breast Cancer Symposium. Abstract PD10-03. Presented December 9, 2021.
4. Schmid P, Im SA, Armstrong A, et al: BEGONIA: Phase 1b/2 study of durvalumab combinations in locally advanced/metastatic triple-negative breast cancer—Initial results from arm 1, durvalumab + paclitaxel, and arm 6, durvalumab + trastuzumab deruxtecan (T-DXd). 2021 ASCO Annual Meeting. Abstract 1023. Presented June 4, 2021.

