The latest analysis of the phase III KEYNOTE-522 trial demonstrated significant improvements in clinical outcomes with pembrolizumab plus chemotherapy vs chemotherapy alone as a neoadjuvant/adjuvant treatment of triple-negative breast cancer.1 This is the first large, randomized, phase III trial to report a statistically significant and clinically meaningful event-free survival result in this population.
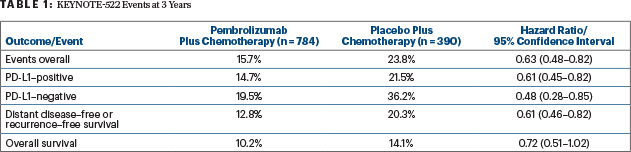
After a median follow-up of 39 months, at the fourth interim analysis, the pembrolizumab arm had a 37% reduction in event-free survival events (hazard ratio [HR] = 0.63; 95% confidence interval [CI] = 0.48–0.82; P = .00031). The 3-year event-free survival rate was 84.5% with pembrolizumab/chemotherapy compared with 76.8% with chemotherapy alone, investigators reported during an ESMO Virtual Plenary presentation.1
“KEYNOTE-522 met its dual primary endpoints,” said lead investigator Peter Schmid, MD, PhD, of the Centre for Experimental Cancer Medicine, Barts Cancer Institute, London. “These results support pembrolizumab plus platinum-containing neoadjuvant chemotherapy, followed by adjuvant pembrolizumab after surgery, as a new standard-of-care treatment regimen for patients with high-risk, early-stage, triple-negative breast cancer.”

Peter Schmid, MD, PhD
Based on these results, on July 27, 2021, the U.S. Food and Drug Administration (FDA) approved pembrolizumab for the treatment of patients with high-risk early-stage triple-negative breast cancer in combination with chemotherapy as neoadjuvant treatment and then continued as a single agent as adjuvant treatment after surgery. The FDA had granted accelerated approval (in November 2020) to pembrolizumab in combination motherapy for patients with locally recurrent unresectable or metastatic triple-negative tumors expressing PD-L1. Based on confirmatory data from KEYNOTE-522, that indication was converted to full approval.
As previously reported, neoadjuvant pembrolizumab plus chemotherapy resulted in a significant increase in pathologic complete response rate (ypT0/Tis ypN0): 64.8% vs 51.2%, an absolute 13.6% improvement (P = .00055).2
“These data have now met statistical significance, with a hazard ratio (HR = 0.63) that is consistent with the previous analysis,” Dr. Schmid announced. At the second interim analysis, event-free survival at 18 months was 91.3% with pembrolizumab plus chemotherapy and 85.3% with chemotherapy alone (HR = 0.63; P = .0089), which did not meet the predefined boundary for significance (P = .00051) at that time (Table 1).
KEYNOTE-522 Design
KEYNOTE-522 enrolled 1,174 patients with stage 2 or 3 early triple-negative breast cancer. More than 80% of patients expressed PD-L1 (ie, Combined Positive Score ≥ 10), and about half had lymph node involvement. Patients were randomly assigned 2:1 to receive one of the two following regimens:
Pembrolizumab plus chemotherapy: Neoadjuvant treatment with pembrolizumab (200 mg every 3 weeks) plus paclitaxel (weekly) and carboplatin (weekly or every 3 weeks) for four cycles, followed by pembrolizumab (200 mg every 3 weeks) plus cyclophosphamide and either doxorubicin or epirubicin (every 3 weeks) for four cycles prior to surgery. This regimen was followed by adjuvant treatment with nine cycles of pembrolizumab (every 3 weeks; n = 784).
Chemotherapy plus placebo: Neoadjuvant treatment with placebo (every 3 weeks) plus paclitaxel (weekly) and carboplatin (weekly or every 3 weeks) for four cycles, followed by placebo plus cyclophosphamide and either doxorubicin or epirubicin (every 3 weeks) prior to surgery. This regimen was followed by adjuvant treatment that included nine cycles of placebo (every 3 weeks; n = 390).
Additional Endpoints
Event-free survival was defined as the time from randomization to the first occurrence of disease progression that precluded definitive surgery, a local/distant recurrence, a second primary cancer, or death from any cause.
Event by category “unfortunately” showed that the majority were distant recurrences, noted Dr. Schmid. These distant recurrences were observed in 13.1% of the chemotherapy arm and 7.7% of the pembrolizumab arm, whereas the rates of local recurrence were 4.4% and 3.6%, respectively. “A substantial proportion of this group” developed subsequent distant recurrences not yet reflected as such, he added.
In prespecified exploratory subgroup analyses, the 3-year event-free survival benefit seen with pembrolizumab was independent of PD-L1 expression (Table 1) or achievement of pathologic complete response. Following a pathologic complete response, event-free survival was 94.4% with pembrolizumab plus chemotherapy and 92.5% with placebo plus chemotherapy; without a pathologic complete response, these rates were 67.4% vs 56.8%, respectively.
“Patients with residual disease have a substantially higher event-free survival if treated with pembrolizumab. In patients achieving a pathologic complete response, we still see a benefit with pembrolizumab of about 2%, suggesting that benefit might be seen irrespective of the achievement of a pathologic complete response,” Dr. Schmid commented.
Currently, overall survival is not mature. However, Dr. Schmid commented, “we can see the beginning separation of the curves” (Table 1).
Tolerability
The safety profile of the pembrolizumab regimen was consistent with the known profiles of each regimen, and no new safety concerns were identified. For the combined neoadjuvant and adjuvant phases, grade 3 or 4 treatment-related adverse events were observed in 77.1% of the pembrolizumab-plus-chemotherapy arm and 73.3% of the chemotherapy-alone arm. Treatment-related toxicity led to death in four patients who received pembrolizumab and in one patient given chemotherapy alone.
“In the adjuvant phase, the incidence of side effects was dramatically lower than in the neoadjuvant phase [which were previously reported],” stated Dr. Schmid. These adverse events occurred in 53.7% of patients receiving pembrolizumab and 48.6% of those given placebo, including 6.3% and 2.7%, respectively, with at least one grade ≥ 3 event and 4.3% and 1.8%, respectively, leading to study discontinuation.
Immune-mediated adverse events and infusion reactions of any grade in the adjuvant phase were observed in 10.2% and 6.0%, respectively. These side effects were grade 3 to 5 in 2.9% and 0.3%, respectively, and led to treatment discontinuation in 1.4% and 0.3%. One patient in the pembrolizumab arm died of autoimmune encephalitis (which may or may not have been related to treatment).
“This is a milestone. We have a positive trial. However, there are still lots of questions…, and we have a rich tissue source to analyze and to understand who benefits more and who benefits less and how to tailor treatment,” Dr. Schmid said.
DISCLOSURE: Dr. Schmid has an immediate family member who has been employed by Genentech and Roche; has received honoraria from AstraZeneca, Novartis, Pfizer, and Roche; has served as a consultant or advisor to AstraZeneca, Bayer, Boehringer Ingelheim, Celgene, Eisai, Merck, Novartis, Pfizer, and Puma Biotechnology; has an immediate family member who has served as a consultant or advisor to Genentech/Roche; and has received institutional research funding from Astellas Pharma, AstraZeneca, Genentech, Novartis, OncoGenex, and Roche.
REFERENCES
1. Schmid P, Cortes J, Dent R, et al: KEYNOTE-522: Phase 3 study of neoadjuvant pembrolizumab plus chemotherapy versus placebo plus chemotherapy, followed by adjuvant pembrolizumab versus placebo for early-stage triple-negative breast cancer. ESMO Virtual Plenary. Abstract VP7-2021. Presented July 15, 2021.
2. Schmid P, Cortes J, Pusztai L, et al: Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020.

