The ASCO Post is pleased to present Hematology Expert Review, an ongoing feature that occasionally quizzes readers on issues in hematology. In this installment, the authors highlight the most common type of systemic amyloidosis in the United States: immunoglobulin light chain [or amyloid light chain] (AL) amyloidosis. For each of the quiz questions that follow the case presentations, select the one best answer. The correct answers and accompanying discussions follow.

GUEST EDITORS
Dr. Abutalib is Associate Director of the Hematology and Cellular Therapy Program and Director of the Clinical Apheresis Program, Cancer Treatment Centers of America, Zion, Illinois; Associate Professor at Rosalind Franklin University of Medicine and Science; and Founder and Co-Editor-in-Chief of Advances in Cell & Gene Therapy. Ms. Mendelson is a nurse practitioner at the Amyloidosis Center, Assistant Professor of Medicine at Boston University School of Medicine, and hematology medical oncology provider at Boston Medical Center. Dr. Sanchorawala is Professor of Medicine and Director of the Amyloidosis Center, Boston University School of Medicine, and Boston Medical Center.
AL amyloidosis has been characterized as a rare disease notable for significant delays in diagnosis, causing vital organ failure and poor outcomes.1,2 Our aim in this short review is to increase awareness of the clinical signs and symptoms associated with AL amyloidosis in patients with multiple myeloma, represent a path to earlier diagnosis, avoid vital organ damage either from the disease or inappropriate anti–plasma cell therapy, and improve overall well-being of the patients.
Case 1: ‘Periorbital Contact Dermatitis’ for About 12 Months
A 56-year-old woman with kappa light chain multiple myeloma was diagnosed in 2013 when she presented with anemia and back pain due to a compression fracture of the T12 vertebral body. She had multiple myeloma with 50% kappa typic plasmacytosis and normal cytogenetics, fluorescence in situ hybridization (FISH) study with t(11;14) translocation, serum and urine electrophoresis, and immunofixation without monoclonal protein. A serum-free kappa light chain level of 1,200 mg/L, serum lambda light chain level of 10 mg/L, and ratio of 120 were noted.
Treatment was initiated with four cycles of lenalidomide, bortezomib, and dexamethasone followed by high-dose melphalan at 200 mg/m2 and autologous hematopoietic cell transplantation (auto-HCT). The patient was then started on lenalidomide maintenance in early 2014. She developed easy bruising around her eyes in the summer of 2014, and after a routine visit with her myeloma specialist, she was referred to a dermatologist. She was diagnosed as having contact dermatitis from eye makeup products. She stopped applying eye makeup, without complete resolution of the bruising.
Meanwhile, the patient remained on lenalidomide maintenance. She started to experience gradual worsening of her breathing and shortness of breath with leg swelling in early 2015. An echocardiogram showed increased left-ventricular wall thickness, interventricular septum measurement of 13 mm, and left-ventricular ejection fraction of 60%. Her cardiac biomarkers were elevated: N-terminal–pro B-type natriuretic peptide (NT-proBNP) was 1,200 pg/mL, BNP was 400 pg/mL, and troponin I was 0.1 ng/mL.
In addition to worsening dyspnea, she now also had more frequent spontaneous periorbital bruising despite not applying eye makeup. A year after “easy bruising” around the eyes, consideration for AL amyloidosis was given in the summer of 2015. An abdominal fat pad aspiration was performed and subjected to Congo red staining, which showed congophilic amyloid deposits (ie, readily stained by Congo red).
Final Diagnosis: A case of multiple myeloma with subsequent development of AL amyloidosis while on maintenance therapy for myeloma after auto-HCT.
Case 2: ‘Seronegative Rheumatoid Arthritis’ for > 24 months
A 54-year-old man was diagnosed with lambda light chain multiple myeloma in 2019. He presented with joint pain, marked reduced range of motion, and multiple tendon ruptures in 2016. He was then seen by his family physician and orthopedic surgeon, who recommended he stop playing hockey, which he sadly did. He was referred to a rheumatology clinic, where serologic evaluation was negative, and he was diagnosed with seronegative rheumatoid arthritis in 2017.
Symptoms and range of motion worsened despite treatment with prednisone, methotrexate, adalimumab, and leflunomide. He eventually had to go on disability. In 2017, he developed persistent periorbital ecchymosis and visited more than 10 different physicians prior to being diagnosed with possible amyloidosis by a dermatologist in 2019, and he was finally referred to a hematologist.
Bone marrow biopsy demonstrated 85% to 90% lambda typic plasmacytosis, with normal cytogenetics and FISH studies. The serum immunofixation electrophoresis disclosed trace IgG lambda, and the urine electrophoresis and immunofixation showed Bence Jones lambda at 3.7 g/24 h. The free light chain assay showed kappa 1.5 mg/L, lambda 3,015 mg/L, and kappa-lambda ratio of 0. A 24-hour urine showed 4,698 mg of protein (of which 3,700 mg was Bence Jones protein), and serum creatinine was 0.9 mg/dL. The BNP level was 172 pg/mL, NT-proBNP level was 795 pg/mL, and troponin I was 0.016 ng/mL. An echocardiogram disclosed an ejection fraction of 65%, interventricular septum measurement of 9 mm (range = 7–11 mm), and global longitudinal strain of –23.8% (normal > –17.1%). An abdominal fat pad aspiration was performed and subjected to Congo red staining, which showed congophilic amyloid deposits confirming the diagnosis.
Final Diagnosis:A case of multiple myeloma–associated AL amyloidosis with debilitating soft-tissue involvement. Initial misdiagnosis of multiple myeloma–associated AL amyloidosis led to inappropriate pharmacologic therapy for seronegative rheumatoid arthritis.
Question 1
What proportion of newly diagnosed patients with multiple myeloma may harbor subclinical AL amyloidosis?
A. 1%–2%
B. 5%−6%
C. 30%−40%
D. 60%−65%
Question 2
Which of the following symptoms may be related to AL amyloidosis in patients with multiple myeloma?
A. Joint pain
B. Constipation
C. Diarrhea
D. All the above
Question 3
Which of the following clinical signs and symptoms may be indicative of soft-tissue involvement by AL amyloidosis in patients with multiple myeloma?
A. Submandibular salivary gland swelling
B. Nonpitting edema of the lower extremities
C. Lymphadenopathy
D. All the above
Answers to Hematology Expert Review Questions
Question 1
What proportion of newly diagnosed patients with multiple myeloma may harbor subclinical AL
amyloidosis?
Correct answer: C. 30%–40%
Expert Perspective
Like multiple myeloma, AL amyloidosis is associated with a plasma cell dyscrasia. However, in AL amyloidosis, there is deposition of amyloid fibrils, derived from immunoglobulin light chains, into a multitude of organs and soft tissues, leading to a plethora of clinical symptoms and eventually vital organ dysfunction. It has been estimated that overt organ dysfunction from AL amyloidosis occurs in approximately 10% of patients with multiple myeloma; however, subclinical or occult AL amyloidosis is higher and may occur in 30% to 40% of patients with multiple myeloma.2-5
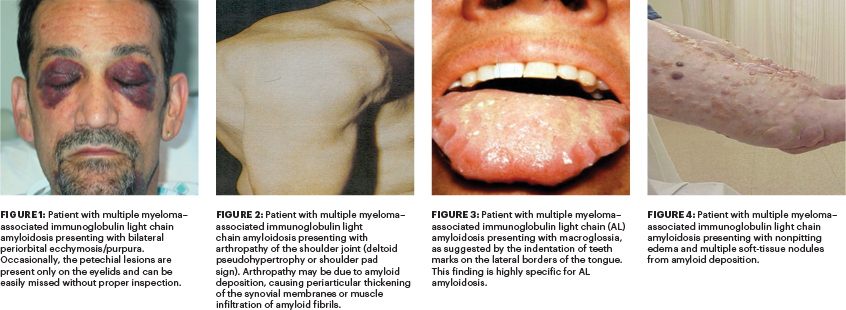
Despite the known relationship between the two disorders, delays in the diagnosis of AL amyloidosis in patients with existing myeloma remains a major problem, as highlighted in the two cases presented in this review. Such delays invariably are associated with needless physical, mental, and financial distress to patients and their families.
Question 2
Which of the following symptoms may be related to AL amyloidosis in patients with multiple myeloma?
Correct answer:D. All the above
Expert Perspective
Most recently, the results of a retrospective review1 of patients seen at the Boston University Amyloidosis Center from 2009 to 2018 with evidence of multiple myeloma based on the International Myeloma Working Group criteria6 and biopsy-proven AL amyloidosis were reported. A total of 992 patients with AL amyloidosis were seen over a 10-year period from 2009 to 2018.1 Of these patients, 79 were classified as myeloma-associated AL amyloidosis, representing 8% of all cases of AL amyloidosis in this series. Of these 79 patients, 33% (n = 26) presented with complaints related to soft-tissue involvement, the most common being joint pain owing to amyloid arthropathy (n = 13), followed by soft-tissue masses (n = 8) and macroglossia (n = 5); 25% (n = 20) presented with cardiac symptoms of dyspnea (n = 10), chest pain (n = 4), edema (n = 2), and syncope (n = 2); 8% presented with fatigue, 7% presented with gastrointestinal complaints, and 5% presented with periorbital ecchymosis.
Question 3
Which of the following clinical signs and symptoms may be indicative of soft-tissue involvement by AL amyloidosis in patients with multiple myeloma?
Correct answer: D. All the above
Expert Perspective
Almost one-half (43%) of patients with myeloma-associated AL amyloidosis present with soft-tissue involvement1 (Figures 1–4, Table 1). This, in contrast, is much higher than in patients with AL amyloidosis alone, where the incidence of soft-tissue involvement is about 23%.7
Given there is no laboratory test to screen for soft-tissue involvement, it is imperative for clinicians to be familiar with findings that are indicative. Some of them are pathognomonic (eg, periorbital ecchymosis/purpura, macroglossia [be sure to inspect the base of the tongue], and deltoid pseudohypertrophy, also known as shoulder pad sign) for soft-tissue involvement by AL amyloidosis in
patients with multiple myeloma. Edema can be pitting or nonpitting in patients with AL amyloidosis (eg, caused by cardiac dysfunction, nephrotic syndrome, or soft-tissue deposition).
DISCLOSURE: Dr. Abutalib is an advisor for AstraZeneca. Ms. Mendelson reported no conflicts of interest. Dr. Sanchorawala has served as a consultant or advisor to AbbVie, Caelum Biosciences, Janssen, and Proclara and has received institutional research funding from Celgene, Janssen, Oncopeptides, Prothena, and Takeda.
REFERENCES
1. Mendelson L, Shelton A, Brauneis D, et al: AL amyloidosis in myeloma: Red flag symptoms. Clin Lymphoma Myeloma Leuk 20:S2152-S2650, 2020.
2. Schulman A, Connors LH, Weinberg J, et al: Patient outcomes in light chain amyloidosis: The clock is ticking from symptoms to diagnosis. Eur J Haematol. June 21, 2020 (early release online).
3. Rajkumar SV, Gertz MA, Kyle RA: Primary systemic amyloidosis with delayed progression to multiple myeloma. Cancer 82:1501-1505, 1998.
4. Desikan KR, Dhodapkar MV, Hough A, et al: Incidence and impact of light chain associated amyloidosis on the prognosis of patients with multiple myeloma treated with autologous transplantation. Leuk Lymphoma 27:315-319, 1997.
5. Bahlis NJ, Lazarus HM: Multiple myeloma-associated AL amyloidosis: Is a distinctive therapeutic approach warranted? Bone Marrow Transplant 38:7-15, 2006.
6. Rajkumar SV, Dimopoulos MA, Palumbo A, et al: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014.
7. Merlini G, Dispenzieri A, Sanchorawala V, et al: Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers 4:38, 2018.

