
Angelo Di Leo, MD
THE VALUE OF cyclin-dependent kinase 4/6 (CDK4/6) inhibitors in advanced breast cancer became ever more certain with the announcement of interim data from MONARCH 3, which evaluated abemaciclib in combination with endocrine therapy as first-line therapy for hormone receptor–positive/HER2-negative advanced breast cancer. The study was reported at the 2017 European Society for Medical Oncology (ESMO) Congress by Angelo Di Leo, MD, of the Istituto Toscano Tuori in Prato, Italy.1
Abemaciclib reduced the risk of disease progression by 46%, with benefit observed across all subgroups but of greater magnitude in some than others, Dr. Di Leo reported. “Our exploratory subgroup analyses suggest that patients with indicators of poor prognosis had substantial benefit from the addition of abemaciclib, whereas in patients with a long treatment-free interval or bone-only disease, single-agent endocrine therapy may remain an appropriate initial therapy,” he said.
Abemaciclib is a small-molecule inhibitor of CDK4 and CDK6, which drive the cell cycle. Two additional CDK4/6 inhibitors, palbociclib (Ibrance) and ribociclib (Kisqali), are approved by the U.S. Food and Drug Administration in combination with endocrine therapy for metastatic breast cancer. Abemaciclib received priority review in July 2017 as monotherapy and in combination with fulvestrant (Faslodex) for previously treated patients with advanced disease.
MONARCH 3 Details
MONARCH 3 IS A PHASE III randomized, double-blind trial of abemaciclib given with a nonsteroidal aromatase inhibitor as initial therapy in 493 postmenopausal women with hormone receptor–positive/HER2-negative advanced breast cancer. Patients received abemaciclib at 150 mg/d on a continuous schedule or placebo, plus endocrine therapy with either anastrozole at 1 mg/d or letrozole at 2.5 mg/d. The primary endpoint was progression-free survival.
“For the first time, we have insights suggesting that patients with certain clinical characteristics may benefit differently from treatment with a CDK4/6 inhibitor….”— Angelo Di Leo, MD
Tweet this quote
Dr. Di Leo presented the interim analysis of MONARCH 3, reporting that the combination of abemaciclib plus endocrine therapy, compared with endocrine therapy alone, significantly prolonged progression-free survival, with a hazard ratio (HR) of 0.543 and a highly significant P value (P = .000021). Median progression-free survival was 14.7 months with endocrine therapy but was not yet reached with the combination therapy. In patients with measurable disease, the objective response rate was 59% vs 44%, respectively (P = .004).
Magnitude of Benefit Varied
THE TREATMENT EFFECT of abemaciclib was consistently observed across subgroups, based on hazard ratios, but patients with good-prognosis factors derived less benefit from the combination therapy. (Table 1).
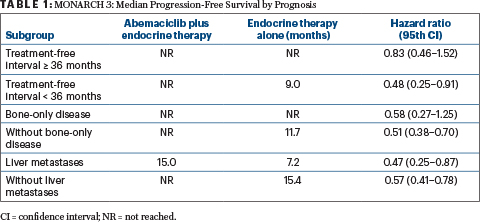
Table 1
At 18 months, the landmark progression-free survival rates with the combination therapy vs placebo were 53.0% and 30.3%, respectively, for patients with a short treatment-free interval; 57.5% vs 35.4% for patients without bone-only disease; and 35.3% and 21.5% for patients with liver metastases.
Toxicity Profile
ADVERSE events were more frequent with the combination therapy. Abemaciclib dosed on a continuous schedule was generally well tolerated. Diarrhea (all grades) occurred in 81.3%, and grade 3 diarrhea was seen in 9.5% of patients on both drugs. This side effect was effectively managed with dose reductions and antidiarrheal medication. Neutropenia (all grades) occurred in 41.3%, and grade 3/4 neutropenia was seen in 21.1%; however, in only one patient was this associated with neutropenic fever.
Other side effects of note with abemaciclib/endocrine therapy include fatigue in 40.1% and nausea in 38.5% (all grades). There was also a greater increase in creatinine levels, but this was not associated with impairment in renal function. Eight deaths occurred in the abemaciclib arm due to adverse events, including deaths from lung infection, embolism, cerebral ischemia, pneumonitis, and respiratory failure, and two deaths occurred in the placebo arm, due to general health deterioration and sudden death.
For Some Patients, Hold the CDK4/6 Inhibitor
ALTHOUGH THE TREATMENT EFFECT of abemaciclib was consistently observed across subgroups, based on hazard ratios, he maintained that the combination therapy is probably not warranted for all patients. “For the first time, we have insights suggesting that patients with certain clinical characteristics may benefit differently from treatment with a CDK4/6 inhibitor…. Our exploratory subgroup analyses suggest that the largest benefit was observed in patients with adverse prognostic factors, such as liver metastases or relapse only a few years after adjuvant therapy,” he revealed.
ABEMACICLIB IN MONARCH 3
- MONARCH 3 evaluated the CDK4/6 inhibitor abemaciclib as initial therapy for hormone receptor–positive, HER2-negative advanced breast cancer.
- Abemaciclib in combination with a nonsteroidal aromatase inhibitor significantly improved progression-free survival. Median progression-free survival was 14.7 months with endocrine therapy alone and was not yet reached with the combination.
- The most benefit was observed in patients with poor-prognosis factors, such as a treatment-free interval less than 36 months.
Therefore, added Dr. Di Leo, patients with good-prognosis factors, such as bone-only disease or a long treatment-free interval, may still be treated with endocrine therapy alone, since their outcomes were already good. Approximately one-third of patients in the study fell into this category—“a clinically relevant proportion of patients for whom we may consider delaying the use of a CDK4/6 inhibitor and thus avoid toxicity and costs,” he commented.
How Abemaciclib Differs From Other CDK4/6 Inhibitors
ALTHOUGH THE THREE CDK4/6 inhibitors have demonstrated similar efficacy in their respective clinical trials, Dr. Di Leo emphasized in a press briefing that there are differences among them. In preclinical models, abemaciclib showed greater affinity for CDK4 than did palbociclib and ribociclib; CDK4 is thought to be more important than CDK6 in breast cancer oncogenesis.2 According to Dr. Di Leo, abemaciclib has a 14-fold greater potency for CDK4 over CDK6, and this may explain why neutropenia occurs less with abemaciclib than with other CDK4/6 inhibitors.
“Additionally, palbociclib and ribociclib are limited by their pharmacodynamics in their on-target inhibition of CDK6, which is critical for hematopoiesis,” stated Neil Vasan, MD, PhD, and Maura N. Dickler, MD, in an article in the August 10, 2017, issue of The ASCO Post. As a result, palbociclib and ribociclib require “off weeks” to allow for bone marrow recovery, whereas abemaciclib can be dosed continuously without a break.
In short, Dr. Di Leo commented: “It’s quite clear that the safety profile of these compounds is not entirely overlapping.” The most salient differences are the higher rates of diarrhea with abemaciclib and more neutropenia with palbociclib and ribociclib. In addition, in the MONARCH 3 trial, the incidence of venous thromboemoblism was higher in the abemaciclib arm (4.9%) than in the control arm (0.6%). The increase in the rate of venous thromboembolism has not been observed in trials with ribociclib and palbociclib. “These differences can be important for clinicians in tailoring treatment according to the individual needs of each patient.” ■
DISCLOSURE: Dr. Di Leo reported no conflicts of interest.
REFERENCES
1. Di Leo A, Toi M, Campone M, et al: MONARCH 3: Abemaciclib as initial therapy for patients with HR+/HER2– advanced breast cancer. 2017 ESMO Congress. Abstract 236O_PR. Presented September 10, 2017.
2. Asghar U, Witkiewicz AK, Turner NC, et al: The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov 14:130-146, 2015.


