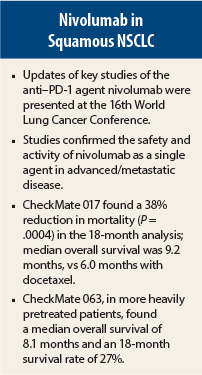
For the treatment of advanced squamous non–small cell lung cancer (NSCLC), the programmed cell death protein 1 (PD-1) antibody nivolumab (Opdivo) continues to show results in key trials that now report 18-month data. The updates were reported at the 16th World Conference on Lung Cancer in Denver, Colorado.
In another trial reported at this meeting, CheckMate 012, the combination of nivolumab plus ipilimumab (Yervoy) showed encouraging activity and less-than-expected toxicity. (The study results were reported in the September 25 issue of The ASCO Post.)
Squamous cell histology constitutes about 20% of NSCLC cases, heralds a poor prognosis, and has few effective treatment options. Second-line docetaxel typically extends survival 6 to 8 months, speakers said at the meeting.
The immune checkpoint inhibitor nivolumab has demonstrated activity in both squamous and nonsquamous NSCLC. It is now approved by the U.S. Food and Drug Administration for patients with squamous NSCLC that progresses during or after platinum-based chemotherapy.
CheckMate 017 at 18 Months
In the 18-month follow-up of the phase III CheckMate 017 trial, involving 272 previously treated patients with advanced squamous NSCLC, nivolumab improved overall survival from 6.0 months with docetaxel to 9.2 months; this represented a 38% reduction in death that was highly significant (P = .0004), reported Karen Reckamp, MD, of City of Hope Comprehensive Cancer Center, Duarte, California.1 “The study shows an unprecedented overall survival at 18 months—28%,” she noted.
“The nivolumab benefit was independent of PD-L1 [ligand of PD-1] expression and was seen across all predefined clinical subgroups,” Dr. Reckamp added. “The safety profile of nivolumab continues to be favorable vs docetaxel and consistent with prior studies.” The majority of patients who developed treatment-related adverse events on nivolumab did so within the first 3 months, she said.
At the 2015 ASCO Annual Meeting, preliminary results from the trial showed a 1-year survival of 42% with nivolumab, vs 24% with docetaxel, and a median progression-free survival of 9.2 vs 6.0 months, respectively.2 The objective response rate was 20% vs 9% (P = .0083), for the nivolumab vs docetaxel arms, respectively.
Longer follow-up has confirmed the superiority of nivolumab over docetaxel, including the primary endpoint of median overall survival. At 18 months, the overall survival rate was 28% vs 13%; the progression-free survival rate was 17% vs 2.7%; the median progression-free survival was 3.5 vs 2.8 months (P = .0008).
The anti–PD-1 antibody was favored across all prespecified subgroups, including age, geographic location, performance status, and prior therapy. “Moreover, the survival benefit was independent of PD-L1 expression,” noted Dr. Reckamp.
Patients treated with nivolumab had substantially fewer treatment-related adverse events, including any adverse event (59% vs 87%), grade 3–5 adverse events (8% vs 58%), and adverse events leading to treatment discontinuation (5% vs 10%). No treatment-related deaths occurred in the nivolumab arm, compared with 2% of docetaxel patients.
Similar Findings From CheckMate 063
Updated analysis of the phase II, single-arm CheckMate 063 trial were reported by Leora Horn, MD, of Vanderbilt-Ingram Cancer Center, Nashville.3 The study involved 117 patients with advanced/metastatic squamous NSCLC that had progressed after at least two prior therapies; 65% of patients had received at least three prior therapies.
The primary endpoint, objective response, was observed in 15% of patients, three-quarters of which were ongoing at analysis. Responses were observed across all predefined subgroups, including age, number of prior therapies, performance status, and level of PD-L1 expression, Dr. Horn reported.
The median time to response was 3.3 months, and the median duration of response has yet to be reached (range, 1.9–11.5 months). Median progression-free survival in this heavily pretreated population was 1.9 months, with a 1-year progression-free survival of 20%. Survival data at 18 months included a median overall survival of 8.1 months, 1-year overall survival rate of 39%, and 18-month survival rate of 27%. Grade 3–4 adverse events occurred in 17% of patients.
As in CheckMate 017, clinical benefit with nivolumab was observed independently of PD-L1 expression in CheckMate 063.
Nivolumab in a ‘Real-Life’ Setting
Maen Hussein, MD, of Florida Cancer Specialists, Lady Lake, Florida, provided data from CA209-153, a largely community-based cancer center trial of 824 previously treated patients with advanced/metastatic squamous and nonsquamous NSCLC.4
Responses were observed in 12% of patients, and 44% achieved stable disease. Grade 3–5 treatment-related adverse events were observed in 8%, and only 2% of patients discontinued treatment as a result.
“Safety analyses are consistent with our prior nivolumab study experience, and no new safety signals have been identified in this trial…. We showed that nivolumab is actually safe to give in community research sites,” Dr. Hussein said.
The study aimed to determine whether patients with a poor performance status suffered more on the drug, and the results were encouraging. The frequency of treatment-related serious adverse events and select adverse events of interest was similar between patients with an Eastern Cooperative Oncology Group performance status of between 0 and 1 and those with a performance status of 2, he reported.
“Early data [median follow-up of 10.4 weeks] from this large multicenter trial suggest that patients with pretreated advanced NSCLC experience clinical benefit from nivolumab therapy regardless of histology type, performance status, EGFR/ALK mutation status, or number of prior therapies,” he said.
“Interestingly, we saw responses in patients with EGFR or ALK mutations, and this has not previously been seen.… We also still saw responses in patients with low PD-L1 expression,” concluded Dr. Hussein. ■
Disclosure: Dr. Reckamp has received research funding from Bristol-Myers Squibb. Dr. Horn disclosed relationships with Bristol-Myers Squibb, Xcovery, Genentech, Merck, and AstraZeneca. Dr. Hussein is on the speakers bureau for Bristol-Myers Squibb.
References
1. Reckamp K, Spigel DR, Rizvi NA, et al: Phase 3 randomized trial (CheckMate 017) of nivolumab vs docetaxel in advanced squamous cell non-small cell lung cancer. 16th World Conference on Lung Cancer. Abstract ORAL02.01. Presented September 7, 2015.
2. Brahmer J, Reckamp KL, Baas P, et al: Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 373:123-135, 2015.
3. Horn L, Rizvi NA, Mazières J, et al: Longer-term follow-up of a phase 2 study (CheckMate 063) of nivolumab in patients with advanced, refractory squamous non-small cell lung cancer. 16th World Conference on Lung Cancer. Abstract ORAL02.03. Presented September 7, 2015.
4. Hussein M, McCleod M, Chandler J, et al: Safety and efficacy of nivolumab in an ongoing trial of a PD-L1+/- patient population with metastatic non-small cell lung cancer. 16th World Conference on Lung Cancer. Abstract ORAL02.02. Presented Septem