Two studies presented at the 16th World Conference on Lung Cancer suggest that high expression of the epidermal growth factor receptor (EGFR), or gene copy number, may indicate potential benefit from EGFR antibodies in squamous cell non–small cell lung cancer (NSCLC). The studies were presented by Roy Herbst, MD, PhD, Ensign Professor of Medicine, Professor of Pharmacology, Chief of Medical Oncology, and Director of the Thoracic Oncology Research Program at Yale School of Medicine, New Haven, and Fred R. Hirsch, MD, PhD, Professor of Medicine and Pathology and Pia and Fred R. Hirsch Chair in Lung Cancer at the University of Colorado, Denver.
Dr. Hirsch provided some context at a press briefing, noting that squamous tumors comprise about 25% of all NSCLCs, tend to be smoking-related, and are characterized by a lack of therapeutic benefits. “In nonsquamous, we have seen lots of progress. In squamous, we have seen practically nothing that is therapeutically implementable,” he said.
Dr. Herbst reported results from the phase III SWOG 0819 trial, which demonstrated some benefit for cetuximab (Erbitux) plus chemotherapy in patients with tumors positive for EGFR by fluorescent in situ immunohistochemistry, especially among patients with squamous histology and patients who did not receive bevacizumab (Avastin).1
“The addition of cetuximab had minimal effect on unselected advanced NSCLC patients, but in fluorescent in situ immunohistochemistry–positive patients, there was a suggestion of benefit, predominantly in squamous cell lung cancer and bevacizumab-inappropriate patients,” Dr. Herbst explained.
Dr. Hirsch reported on a correlative analysis of the SQUIRE trial, showing that gain in EGFR gene copy number was associated with a trend for more favorable hazard ratios for overall survival among patients receiving necitumumab plus chemotherapy.2
“Pooling these two studies together, evaluating antibodies in the same family, we find that gene copy number detected by fluorescent in situ immunohistochemistry seems to predict a better outcome and could, in the future, be used for selecting patients for treatment with EGFR antibodies,” Dr. Hirsch said at a press briefing.
SWOG 0819 Details
Dr. Herbst and colleagues assessed the addition of cetuximab to chemotherapy (carboplatin/paclitaxel, plus or minus bevacizumab) in 1,333 newly diagnosed patients with stage IV NSCLC. The study’s co-primary endpoints were progression-free survival in fluorescent in situ immunohistochemistry–positive patients (n = 400) and overall survival in the whole study population. Patients were stratified not only by EGFR status but also according to their receipt of bevacizumab (bevacizumab appropriate vs inappropriate).
They found no benefit in overall survival for the entire population (hazard ratio [HR] = 0.94, P = .34) and no benefit in progression-free survival in the fluorescent in situ immunohistochemistry–positive population (HR = 0.91, P = .37). As a secondary endpoint, Dr. Herbst and colleagues did find a suggestion of a survival benefit in the entire EGFR fluorescent in situ immunohistochemistry–positive population, but it was not statistically significant (HR = 0.83, P = .10).
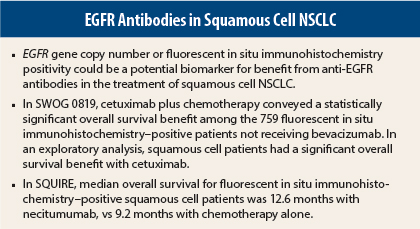
A statistically significant overall survival benefit, however, was seen among the 759 bevacizumab-inappropriate, fluorescent in situ immunohistochemistry–positive patients (HR = 0.75, P = .048). In this group, median overall survival was 8.7 months with chemotherapy alone vs 11.2 months in the cetuximab-containing arm.
In addition, in an exploratory analysis, the study showed a statistically significant overall survival benefit in patients with squamous cell NSCLC receiving cetuximab (11.8 months vs 6.4 months), with a strong hazard ratio (HR = 0.56, P = .006).
The findings suggest that patients with squamous cell lung cancer who have tumors positive for EGFR gene copy number have a survival benefit, whereas those with nonsquamous histology and those with fluorescent in situ immunohistochemistry–negative tumors derive no survival advantage, concluded Dr. Herbst.
“These data, along with the recent SQUIRE results [which evaluated necitumumab], suggest a role for EGFR fluorescent in situ immunohistochemistry in selecting patients [with squamous histology] for therapy with EGFR antibodies, especially when bevacizumab is not used,” Dr. Herbst said. “Analysis of EGFR H Score and KRAS mutation is ongoing, to be presented at ASCO 2016.”
Similar Findings From SQUIRE
The potential for EGFR gene copy number to serve as a biomarker for EGFR antibodies was supported by correlative analysis of the ongoing SQUIRE trial. SQUIRE evaluated the addition of necitumumab to gemcitabine/cisplatin in 1,093 patients with stage IV squamous NSCLC.
Median overall survival was significantly longer in the necitumumab arm than in the chemotherapy-alone arm: 11.5 months vs 9.9 months (HR = 0.84, P = .01).3 As a result of these findings, an advisory board to the U.S. Food and Drug Administration has recommended approval of necitumumab, Dr. Hirsch indicated.
Among the 51% of patients with archived tumor tissue, 37.3% had an increased EGFR gene copy number or were fluorescent in situ immunohistochemistry–positive. Dr. Hirsch presented data regarding the relationship between EGFR protein and EGFR gene copy number and outcomes.
The analysis showed no consistent trends or clear thresholds for a relationship between overall or progression-free survival and the EGFR protein over a range of immunohistochemistry values. However, gain in EGFR gene copy number was associated with a trend for more favorable hazard ratios for overall survival, Dr. Hirsch reported.
For overall survival, hazard ratios related to necitumumab were 0.70 for fluorescent in situ immunohistochemistry–positive patients and 1.02 for fluorescent in situ immunohistochemistry–negative patients. Median overall survival for fluorescent in situ immunohistochemistry–positive patients was 12.6 vs 9.2 months (interaction P value = .066), for the necitumumab vs control arms, respectively.
For progression-free survival, the hazard ratio was 0.71 for fluorescent in situ immunohistochemistry–positive patients, reflecting 6.1 vs 5.1 months (interaction P value = .066), respectively. The hazard ratio for the fluorescent in situ immunohistochemistry–negative group was 1.04.
These were favorable trends observed with necitumumab among the fluorescent in situ immunohistochemistry–positive cohort that were of borderline statistical significance, Dr. Hirsch noted, commenting, “The data from a statistical point of view is not statistically significant. But is it clinically meaningful? I would say yes.” ■
Disclosure: Drs. Herbst and Hirsch reported no potential conflicts of interest.
References
1. Herbst R, Redman MW, Kim ES, et al: A randomized, phase III study comparing carboplatin/paclitaxel or carboplatin/paclitaxel/bevacizumab with or without concurrent cetuximab in patients with advanced non-small cell lung cancer (NSCLC): SWOG S0819. 16th World Conference on Lung Cancer. Abstract PLEN04.01. Presented September 9, 2015.
2. Hirsch FR, Boyle TA, Thatcher N, et al: EGFR IHC and FISH correlative analyses (SQUIRE trial): Necitumumab + gemcitabine-cisplatin vs gemcitabine-cisplatin in 1st-line squamous NSCLC. 16th World Conference on Lung Cancer. Abstract ORAL32.05. Presented September 9, 2015.
3. Thatcher N, Hirsch FR, Luft AV, et al: Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first-line therapy in patients with stage IV squamous non-small-cell lung cancer (SQUIRE): An open-label, randomised, controlled phase 3 trial. Lancet Oncol 16:763-774, 2015.