The addition of durvalumab to neoadjuvant chemotherapy led to higher rates of R0 resections and posed no apparent impediments to successful surgery for resectable non–small cell lung cancer (NSCLC), according to findings from the randomized phase III AEGEAN trial presented at the International Association for the Study of Lung Cancer 2023 World Conference on Lung Cancer (WCLC) in Singapore.1 An interim analysis of AEGEAN previously found a highly significant 32% reduction in events with durvalumab added to the regimen.2
“The AEGEAN trial demonstrated a clinically meaningful improvement in event-free survival with the addition of perioperative durvalumab to neoadjuvant chemotherapy, alongside significantly improved pathologic complete response rates and major pathologic responses. Regardless of disease stage, the addition of perioperative durvalumab to neoadjuvant chemotherapy did not adversely impact the feasibility, type, approach, or timing of surgery in patients with resectable NSCLC and resulted in numerically higher R0 resection rates,” said Tetsuya Mitsudomi, MD, of the Department of Surgery, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.

Tetsuya Mitsudomi, MD
The event-free survival and pathologic complete response rates were previously reported at the American Association for Cancer Research Annual Meeting 2023.2 At the 2023 WCLC, Dr. Mitsudomi focused on the key surgical outcomes. The finding of improved clinical outcomes with a manageable surgical safety profile renders this perioperative regimen a “potential new treatment option” for resectable NSCLC, Dr. Mitsudomi said.
About AEGEAN
The AEGEAN trial is a double-blind, placebo-controlled phase III study that evaluated the use of perioperative durvalumab in combination with neoadjuvant chemotherapy compared with neoadjuvant chemotherapy alone. The trial involved 802 adults with treatment-naive resectable stage II or III NSCLC and a good performance status. Patients were randomly assigned to receive either neoadjuvant durvalumab at 1,500 mg or placebo along with platinum-based chemotherapy for four cycles prior to surgery. Following surgery, patients received durvalumab at 1,500 mg or placebo, respectively, for 12 cycles.
The primary endpoints of the trial were pathologic complete response and event-free survival by blinded independent central review. Efficacy analyses were performed in the modified intent-to-treat population, which comprised 740 patients with no documented EGFR mutations or ALK gene rearrangements.
At enrollment, lobectomy, sleeve resection, and bilobectomy were allowed as the planned surgical procedure. The protocol was amended with enrollment ongoing to exclude patients with tumors classified as T4 for any reason other than size (> 7 cm) or whose planned surgery was pneumonectomy (which was originally allowed), or who had documented test results that confirmed the presence of an EGFR mutation (confirmed by central testing) or ALK translocation (confirmed by local or central testing).
Results of AEGEAN
In a previously reported interim analysis of 402 patients followed a median of 11.7 months, event-free survival was significantly improved with the addition of durvalumab (hazard ratio [HR] = 0.68; P = .003902), based on a median not reached in the durvalumab arm and 25.9 months with chemotherapy alone.2 At 24 months, event-free survival rates were 63.3% and 52.4%, respectively. With the addition of perioperative durvalumab, pathologic complete response rate was increased from 4.3% with chemotherapy alone to 17.2%, an absolute difference of 13% (P = .000036). Major pathologic response was achieved in 33.3% vs 12.3%—a 21% difference (P = .000002).
For the current analysis of 740 patients in the modified intent-to-treat population, all patients in the durvalumab arm received neoadjuvant therapy, as did all but 3 patients in the placebo arm. In both arms, almost 81% underwent surgery, and 77% of each arm completed surgery. Disease progression was the most common reason for cancelled surgery. The key surgical outcomes are shown in Table 1.
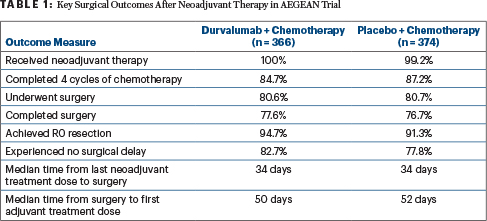
Among patients who underwent surgery, similar proportions in the durvalumab and placebo arms had open procedures (49.2% vs 50.7%) and minimally invasive procedures (49.2% vs 47.0%); lobectomy (including sleeve resection and bilobectomy) was the most common procedure (88.1% vs 85.4%), followed by pneumonectomy (9.2% vs 9.6%). Among patients completing surgery, 86.6% in the durvalumab arm and 84.7% in the placebo arm had mediastinal lymph node dissections.
No Clear Benefit in EGFR-Mutated Subset
Also at the conference, David Harpole, MD, of Duke University Medical Center, Durham, North Carolina, presented findings for the subset of 51 patients with EGFR mutations who entered the study between 2019 and 2021, before patients with EGFR-mutated disease were excluded.3 AEGEAN was initiated before research revealed that patients with EGFR and ALK aberrations have a limited response to immunotherapy.

David Harpole, MD
Perioperative durvalumab plus neoadjuvant chemotherapy demonstrated a limited, nonsignificant benefit in this group. Median event-free survival was 30.8 months vs 19.6 months with placebo (HR = 0.86; 95% CI = 0.35–2.19). At 24 months, 59.3% vs 44.9%, respectively, were event-free. Pathologic complete responses were observed in 3.8% vs 0%, and major pathologic responses were seen in 7.7% and 4.0%, respectively. These numerical differences were deemed not clinically important.
“These findings should be interpreted with caution due to the small number of patients in the EGFR-mutated subgroup and the wide 95% confidence intervals associated with the efficacy results,” Dr. Harpole said. “With the robust clinical benefit demonstrated by adjuvant osimertinib in the phase III ADAURA trial for patients with EGFR-mutated resected NSCLC, mutation testing should be considered prior to starting neoadjuvant therapy.”
Surgical Tolerability
The regimen’s surgical safety profile was good. In the postoperative period, approximately 40% of each arm had an adverse event of any grade possibly related to surgery and about 60% experienced a surgical complication. The most common complaint was procedural pain, which was reported by about 11% of each arm. There were six deaths(2.0%) in the durvalumab arm and four deaths (1.3%) in the placebo arm in the postoperative period.
DISCLOSURE: Dr. Mitsudomi has had financial relationships with AstraZeneca, Chugai, MSD, Ono, Bristol Myers Squibb, Eli Lilly, Novartis, Taiho, Merck, Pfizer, Amgen, Daiichi Sankyo, Takeda, Guardant Health, Boehringer Ingelheim, Thermofisher, Merck Biopharma, and Regeneron. Dr. Harpole has served on the advisory board of AstraZeneca.
REFERENCES
1. Mitsudomi T, Heymach JV, Reck M, et al: Surgical outcomes with neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in resectable NSCLC (AEGEAN). 2023 World Conference on Lung Cancer. Abstract OA12.05. Presented September 11, 2023.
2. Heymach JV, Harpole D, Mitsudomi T, et al: Perioperative durvalumab for resectable non–small-cell lung cancer. N Engl J Med 389:1672-1684, 2023.
3. He J, Gao S, Reck M, et al: Neoadjuvant durvalumab + chemotherapy followed by adjuvant durvalumab in resectable EGFR-mutated NSCLC (AEGEAN). 2023 World Conference on Lung Cancer. Abstract OA12.06. Presented September 11, 2023.

