Real-world outcomes often fall short of those achieved in clinical trials, but this is apparently not so for patients receiving chemoradiotherapy plus consolidation with durvalumab in unresectable stage III non–small cell lung cancer (NSCLC). The robust results achieved in the phase III PACIFIC trial1—which documented a statistically significant 45% reduction in disease progression and 28% reduction in death, ushering in a new standard of care for these patients—are being replicated in clinical practice, according to research presented at the International Association for the Study of Lung Cancer 2023 World Conference on Lung Cancer (WCLC).
Canadian Study: RELEVANCE
Following the approval of durvalumab in Canada, “Canadian medical oncologists expressed the need to understand the implementation of the PACIFIC trial results in the real world,” including real-world treatment patterns and clinical outcomes, said Paul Wheatley-Price, MD, of Ottawa Hospital Research Institute in Canada.2 To this end, he and his co-investigators established the observational, retrospective, cohort RELEVANCE study at five cancer centers.

Paul Wheatley-Price, MD
“We saw almost a tripling of the benefit in progression-free survival and time to next treatment, with the addition of durvalumab to chemoradiotherapy in the real-world setting,” he said. “This real-world retrospective study—which obviously has limitations because of the way we all practice slightly differently and report outcomes slightly differently—really does confirm the benefits of the addition of durvalumab as seen in PACIFIC, particularly in the PD-L1–high population.”
The study evaluated 342 patients with stage III NSCLC treated with chemoradiotherapy plus durvalumab and 155 treated with chemoradiotherapy alone (before the approval of durvalumab) between November 1, 2017, and December 31, 2019. Based on baseline characteristics, Dr. Wheatley-Price indicated that 86% of the patients in RELEVANCE would have been eligible for the PACIFIC trial and therefore reflected the study population. “The PACIFIC-eligible cohort matched the overall cohort of PACIFIC,” he noted. The primary outcome was overall survival (time from chemoradiotherapy to death).
As in PACIFIC, the addition of durvalumab consolidation therapy notably improved median overall survival over chemoradiotherapy alone. Median overall survival was 44.6 months with the addition of durvalumab and 21.3 months with chemoradiotherapy alone. In patients who received durvalumab, significantly longer survival was observed in patients with PD-L1 ≥ 50% vs PD-L1 < 1% (hazard ratio [HR] = 0.24; P < .001). There was no significant difference in survival between patients with PD-L1 expression of 1%–49% and those with PD-L1 < 1% (HR = 0.82; 95% confidence interval [CI] = 0.42–1.63). “The PD-L1 ‘unknown’ population actually did quite well with the addition of durvalumab, which was also seen in some of the supplementary reports from PACIFIC…. The small sample size [of these subsets] and a high proportion of those with unknown PD-L1 expression (32%) limit the interpretation of these results,” he acknowledged.
Median real-world progression-free survival was 24.6 months in the durvalumab arm and 9.6 months in the control arm. Median time to next treatment was 28.6 months and 11.7 months, respectively. No new safety signals were identified in association with the regimen. The most common adverse event of special interest was pneumonitis/interstitial lung disease, with an incidence of 20% (all grades).
“Our study also supports the use of real-world data to validate, in broader populations, the results we see in randomized clinical trials,” he added.
Australian Study
The aim of a study presented by Australian investigators was to audit real-world survival outcomes and postprogression therapy at a single Australian institution.3 “Our study showed that comparable survival outcomes to the PACIFIC trial can be achieved in a real-world setting, despite the majority of patients being treated outside of trial criteria regarding timing of durvalumab commencement,” said Nicole Pringle, MD, of Princess Alexandra Hospital, Queensland, Australia.
The researchers reviewed the records of 87 patients with stage IIII NSCLC treated with chemoradiotherapy and consolidation treatment with durvalumab between June 2017 and December 2021. Baseline characteristics were noted, and clinical outcomes were assessed with independent t-tests—statistical analyses used to compare the means of two groups, to determine whether treatment had an effect in a particular group. Survival outcomes were descriptively compared with historical internal controls, which were patients with stage III NSCLC at the same institution treated between 2009 and 2015, before durvalumab was approved.
At a median follow-up of 23 months, 56% of the patients had disease progression, 34% had died, and 20% had discontinued durvalumab because of adverse events. The median progression-free survival was 22.3 months, and median overall survival was 43.7 months. Age, performance status, PD-L1 expression, and early discontinuation or timing of durvalumab commencement were not significantly associated with any difference in these outcomes. The first site of relapse was intrathoracic for 45%, extrathoracic for 32%, and intracranial for 23% of patients.
“The historical internal cohort of 172 patients had a median overall survival of 25.4 months. This pointed to improved overall survival of patients since the introduction of durvalumab,” Dr. Pringle said.
The outcomes mirrored those of PACIFIC, despite the fact that only 20% of patients started durvalumab within 6 weeks of chemotherapy, which is recommended.
PACIFIC-R: Real-World Benefit Limited to EGFR Wild-Type
The real-world benefit of the PACIFIC regimen—comparable to the clinical trial results—was recently shown in the PACIFIC-R trial,4 a study of patients who received durvalumab through the global PACIFIC early access program. In PACIFIC, the chemoradiotherapy/durvalumab regimen was not superior to chemotherapy alone in patients with mutations of the epidermal growth factor receptor (EGFR).5 Whether this is also true in the real-world setting was the subject of an analysis of PACIFIC-R presented at the World Conference by Solange Peters, MD, PhD, of the Centre Hospitalier Universitaire Vaudois, Lausanne, Switzerland.6

Solange Peters, MD, PhD
In that analysis, PACIFIC-R patients with EGFR mutations derived less benefit with regard to progression-free survival than their wild-type counterparts treated with chemoradiotherapy and durvalumab consolidation, but overall survival was similar between those subsets of patients, Dr. Peters reported. “Outcomes with durvalumab among patients with EGFR-mutated NSCLC in PACIFIC-R were broadly comparable to those of the corresponding subgroup in the PACIFIC trial,” she said.
The lesser benefit in EGFR-mutated patients was also consistent with a number of studies reported at the WCLC showing the activity of immunotherapy to be limited in patients with EGFR mutations.7,8
About PACIFIC-R
PACIFIC-R is an international, observational study conducted as a retrospective review of established medical records of patients with unresectable, stage III NSCLC treated with durvalumab after chemoradiotherapy within an AstraZeneca-initiated early access program between September 2017 and December 2018. The primary endpoints were investigator-assessed real-world progression-free survival and overall survival. Outcome according to EGFR status was exploratory.
The full analysis set comprised all 1,154 patients from 10 countries. Overall, 466 patients had known EGFR status, of which 44 (9.4%) had EGFR-mutated disease, and 422 had EGFR wild-type (90.6%). Clinical characteristics were generally similar between patients with EGFR-mutated and wild-type tumors, with some exceptions: a lower proportion in the EGFR-mutated group were aged < 70 years, had a history of smoking, and had a performance status of 0. In both EGFR-mutated and wild-type subsets, 76% of patients with PD-L1 testing had PD-L1 expression ≥1%.
Not surprisingly, real-world median progression-free survival was lower in the EGFR-mutated group vs the EGFR–wild-type group, but overall survival was similar between the groups (Table 1). Within the EGFR-mutated group, EGFR inhibitors were administered as the first subsequent treatment after durvalumab in 15 of 20 patients with distant metastases, she said.
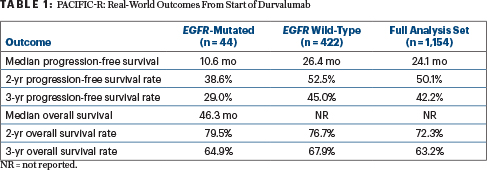
In addition, the time to first subsequent therapy was shorter among patients with EGFR mutations (median = 16.7 vs 43.1 months). Approximately 45% of patients with EGFR mutations developed distant metastases, and 75% of them received an EGFR-targeted tyrosine kinase inhibitor as their first subsequent therapy, she added.
“These data should be interpreted carefully due to the small number of patients with identified EGFR mutations and the PACIFIC-R study’s retrospective nature,” Dr. Peters cautioned.
DISCLOSURE: Dr. Wheatley-Price reported financial relationships with AstraZeneca, Bristol Myers Squibb, Merck, Amgen, Eli Lilly, Novartis, Sanofi, Pfizer, Guardant, Janssen, Jazz Pharmaceuticals, Takeda, and Bayer. Dr. Pringle reported no conflicts of interest. Dr. Peters reported financial relationships with AbbVie, Amgen, Aacus, AstraZeneca, Bayer, BeiGene, BerGenBio, Biocartis, BioInvent, Blueprint Medicines, Boehringer Ingelheim, Bristol Myers Squibb, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, F-Star, Foundation Medicine, Genzyme, Gilead Sciences, GSK, Hutchmed, Illumina, Incyte, Ipsen, iTeos, Janssen, Merck Sharp and Dohme, Merck Serono, Mirati, Novartis, Novocure, Pharma Mar, Promontory Therapeutics, Pfizer, Regeneron, Roche/Genentech, Sanofi, Seattle Genetics, Takeda, Vaccibody, AiCME, Fishawack, Genzyme, Gilead Sciences, Imedex, IQVIA, Merrimack, Oncology Education, PER, PeerView, RMEI, RTP, and Galencia.
REFERENCES
1. Antonia SJ, Villegas A, Daniel D, et al: Durvalumab after chemoradiotherapy in stage III non–small-cell lung cancer. N Engl J Med 377:1919-1929, 2017.
2. Wheatley-Price P, Navani V, Pabani A, et al: Real-world survival with CRT + durvalumab for unresectable, stage III NSCLC in Canada: The RELEVANCE study. 2023 World Conference on Lung Cancer. Abstract MA16.06. Presented September 12, 2023.
3. Pringle N, Flether JA, Morton C, et al: Real world experience of survival outcomes in consolidative durvalumab in unresectable stage III non-small cell lung cancer. 2023 World Conference on Lung Cancer. Abstract EP08.02-03. Presented September 7, 2023.
4. Girard N, Bar J, Garrido P, et al: Treatment characteristics and real-world progression-free survival in patients with unresectable stage III NSCLC who received durvalumab after chemoradiotherapy: Findings from the PACIFIC-R study. J Thorac Oncol 18:181-193, 2023.
5. Naidoo J, Antonia S, Wu YL, et al: Durvalumab after chemoradiotherapy in unresectable stage III EGFR-mutant NSCLC: A post hoc subgroup analysis from PACIFIC. J Thorac Oncol 18:657-663, 2023.
6. Peters S, Christoph DC, Field JK, et al: Next steps in locally advanced NSCLC: Optimizing techniques & choosing populations that benefit (PACIFIC-R). 2023 World Conference on Lung Cancer. Abstract OA17.03. Presented September 11, 2023.
7. Zhou C, Dong X, Chen G, et al: Impower151: Phase III study of atezolizumab + bevacizumab + chemotherapy in first-line metastatic nonsquamous NSCLC. 2023 World Lung Cancer Conference. Abstract OA09.06. Presented September 11, 2021.
8. Lee C, Liao BC, Subramaniam S, et al: ILLUMINATE: Efficacy and safety of durvalumab-tremelimumab and chemotherapy in EGFR mutant NSCLC following progression on EGFR inhibitors. 2023 World Lung Cancer Conference. Abstract OA09.04. Presented September 11, 2021.

