In the international phase III EPOCH trial, patients with colorectal liver metastases who experienced disease progression on first-line therapy derived significant benefit from treatment with transarterial yttrium Y-90 radioembolization in combination with systemic chemotherapy, according to Mary F. Mulcahy, MD, of Northwestern University, Chicago.1 The study findings were simultaneously published in the Journal of Clinical Oncology.2

Mary F. Mulcahy, MD
“EPOCH is the first positive phase III global study of transarterial Y-90 radioembolization and chemotherapy in the second-line treatment of patients with colorectal liver metastases. The addition of transarterial Y-90 radioembolization to second-line systemic therapy for colorectal liver metastases resulted in significantly longer progression-free survival and hepatic progression-free survival,” Dr. Mulcahy reported. “This is the first arterial radiotherapy device to demonstrate a statistically significant delay in progression of metastatic colorectal cancer.”
Transarterial radioembolization with Y-90 glass microspheres provides selective internal radiotherapy, with beta-emitting particles delivered through the hepatic vasculature into tumor-feeding arteries. The inherent vascularity of hepatic colorectal lesions in the arterial phase provides a strong rationale for the investigation of glass-based transarterial radioembolization in the second-line setting, she explained.
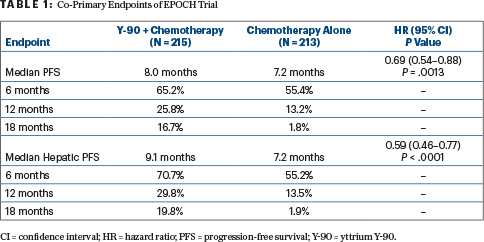
Transarterial radioembolization with Y-90 plus chemotherapy resulted in a median progression-free survival of 8.0 months, vs 7.2 months with chemotherapy alone (hazard ratio [HR] = 0.69; P = .0013). Hepatic progression-free survival was increased as well to 9.1 months, vs 7.2 months (HR = 0.59; P < .0001). However, no significant differences were seen in overall survival.
About EPOCH
The EPOCHtrial enrolled 428 patients with unresectable unilobar or bilobar liver metastases who were fit for chemotherapy (irinotecan- or oxaliplatin-based), with or without targeted therapy, as second-line treatment. The liver tumor burden was less than 10% in more than half the patients, and more than two-thirds of patients had a maximum lesion size of at least 40 mm. Approximately two-thirds had at least 6 lesions, and more than one-third had at least 10 lesions. About half the study patients had nonmetastatic extrahepatic lesions at baseline. Almost half the patients had KRAS mutations.
Patients were randomly assigned 1:1 to receive chemotherapy alone or chemotherapy plus transarterial radioembolization with Y-90 glass microspheres. For that arm, patients received chemotherapy in cycle 1, transarterial radioembolization with Y-90 in cycle 2 (replacing chemotherapy, for one cycle), and chemotherapy with or without targeted therapy in cycle 3. They were followed every 8 weeks until disease or hepatic disease progression.
Second-line therapy was irinotecan-based in 60%; biologics were part of treatment for 42%; bevacizumab was given to 42%, and less than 5% received an agent targeting epidermal growth factor receptor.
Co-primary endpoints were progression-free survival and hepatic progression-free survival by blinded independent central review. Statistical significance was set at a one-sided alpha level of 0.00248.
Primary Endpoints Met
The study met its two primary endpoints, significantly improving progression-free and hepatic progression-free survival (Table 1).
In key subgroups, the transarterial radioembolization/chemotherapy approach yielded considerable improvement in progression-free survival in patients with KRAS mutations (HR = 0.57), left-sided primaries (HR = 0.65), hepatic tumor burden of between10% and 25% (HR = 0.43), and fewer than three lesions (HR = 0.33), as well as in patients receiving a biologic as part of second-line treatment (HR = 0.58). Outcomes for hepatic progression-free survival mirrored these findings.
Median overall survival was 14.0 months in the combination arm and 14.4 months in the chemotherapy arm (HR = 1.07; P = .7229). Approximately one-third of patients in each arm were alive at 18 months.
Overall response rates were 34.0% with transarterial radioembolization/chemotherapy and 21.1% with chemotherapy alone. Stable disease was observed in 45.6% and 51.6%, respectively. Approximately 13% of patients in both arms had progressive disease as best response.
Safety Profile
According to Dr. Mulcahy, Y-90 did not compromise the ability to receive additional chemotherapy, and chemotherapy-related adverse events were similar between the groups. Treatment-emergent adverse events requiring discontinuation of chemotherapy were similar between the arms, occurring in about 12% of patients.
However, 55% of those given transarterial radioembolization had an adverse event related to the device, and 29% had an angiographic procedure–related adverse event. Serious treatment-emergent adverse device events were observed in 20 patients (10.7%) in the transarterial radioembolization group, and 8 of them (4.3% overall) had a fatal outcome.
DISCLOSURE: Dr. Mulcahy has received institutional research funding from Boston Scientific.
REFERENCES
1. Mulcahy MJ, Salem R, Mahvash A, et al: Radioembolization with chemotherapy for colorectal liver metastases: A randomized, open-label, international, multicenter, phase III trial (EPOCH study). ESMO Congress 2021. Abstract LBA21. Presented September 20, 2021.

