More than one out of two patients with metastatic melanoma treated with the combination of nivolumab plus ipilimu-mab is still alive after 5 years, according to the longest follow-up of patients receiving this combination. In two additional studies, the immunotherapy duet also proved to be active in patients with brain metastases, as well as in those with no evidence of disease after resection, according to studies reported at the European Society for Medical Oncology (ESMO) Congress 2019.

James Larkin, PhD
“These are major improvements on what we have seen historically,” said lead investigator of CheckMate 067, James Larkin, PhD, of the Royal Marsden Hospital NHS Foundation Trust, London. As he noted, a decade ago, about 5% of patients reached this landmark, rising to just about 20% with single-agent immunotherapy. Studies reported at the ESMO Congress validate the successes that can be achieved with this regimen.1–3
CheckMate 067: More Than Half of Patients Alive at 5 Years
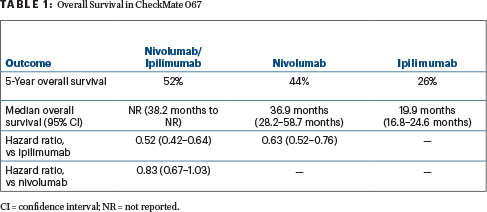
In the 5-year results of the CheckMate 067 trial, both nivolumab alone or in combination with ipilimumab provided significant improvements in overall survival, progression-free survival, and objective response rates over ipilimumab alone in patients with advanced treatment-naive melanoma.1 The combination yielded the most impressive outcomes, with 52% of patients still alive at 5 years, reported Dr. Larkin.
“At 5 years, the median overall survival was not yet reached for nivolumab (1 mg/kg) plus ipilimumab (3 mg/kg), representing the only treatment for metastatic melanoma for which median survival exceeded 60 months,” said Dr. Larkin. “Separation of the survival curves indicate continued benefit for nivolumab/ipilimumab over nivolumab.”
This 5-year analysis is the longest follow-up of a phase III trial of a checkpoint inhibitor combination to date, the authors noted. The findings were simultaneously published in The NewEngland Journal of Medicine.4
Study Details
The study randomly assigned 945 treatment-naive patients with stage III or IV melanoma to one of three groups: 1) nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg every 3 weeks for 4 doses, followed by nivolumab at 3 mg/kg every 2 weeks; 2) nivolumab at 3 mg/kg every 2 weeks plus ipilimumab-matched placebo; or 3) ipilimumab at 3 mg/kg every 3 weeks for 4 doses plus nivolumab-matched placebo.
The co-primary endpoints were overall survival and progression-free survival. The trial was not designed to compare the nivolumab treatment arms; therefore, outcomes for this comparison are descriptive analyses.
Updated Outcomes
Dr. Larkin reported the overall survival data after a minimum of 60 months of follow-up (Table 1). Both the nivolumab-containing arms improved overall survival, irrespective of BRAF mutation status or baseline dehydrogenase levels; a small association with programmed cell death ligand 1 expression was observed.
The objective response rates for nivolumab/ipilimumab, nivolumab alone, and ipilimumab alone were 58%, 45%, and 19%, respectively. The median duration of response was not reached in either nivolumab arm but was 14.4 months with ipilimumab. “Although the response rate has remained stable, the rates of complete response have increased over the 3-, 4-, and 5-year analyses,” Dr. Larkin added.
The median progression-free survival was 11.5 months, 6.9 months, and 2.9 months, respectively, with 5-year rates of 36%, 29%, and 8%. The combination also delayed the time to subsequent therapy and led to a treatment-free interval of 18.1 months, vs less than 2 months in the other two arms.
“A higher proportion of patients are alive and treatment-free at 5 years with nivolumab/ipilimumab (74%), with no loss in quality of life,” said Dr. Larkin.
IMMUNED: Patients With Fully Resected Stage IV Disease
In theadjuvant setting, the three-arm IMMUNED trial evaluated nivolumab/ipilimumab or single-agent nivolumab, compared with placebo, in patients with fully resected or irradiated stage IV disease deemed to be at high risk for recurrence but with no evidence of disease after surgery.2 Both arms significantly reduced the risk of recurrence, but the benefit was more striking with the combination: a 77% relative risk reduction (P < .0001), according to Dirk Schadendorf, MD, of the University Hospital Essen, Germany.

Dirk Schadendorf, MD
“This is the first prospective randomized placebo-controlled clinical study in patients with stage IV melanoma who have with no evidence of disease,” he said. “We saw that the clinical benefit of nivolumab plus ipilimumab is present despite a treatment discontinuation rate of more than 79% and a maximum dose of two infusions for 50% of the patients.”
Although adjuvant therapy is known to be beneficial in patients deemed to be at risk for recurrence after surgery, IMMUNED has a unique feature:Iit enrolled patients with no evidence of disease. “Patients with resected stage IV melanoma and no evidence of disease have generally been excluded from phase III trials in the metastatic setting. They were also not included in recent adjuvant therapy phase III trials, as these studies generally focused on stage III disease—with the exception of CheckMate 238 (20% of patients had resected stage IV melanoma),” he said. (Discussion of CheckMate 238 results follow.)
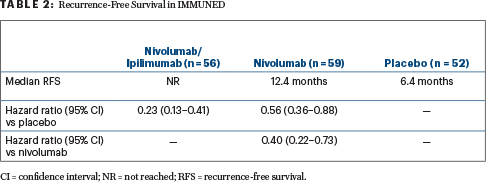
The 167 patients with high-risk stage IV disease in IMMUNED were randomly assigned after surgery to nivolumab at 3 mg/kg (with maintenance nivolumab), nivolumab at 1 mg/kg plus ipilimumab at 3 mg/kg (with maintenance nivolumab), or placebo. Recurrence-free survival was the primary endpoint.
Although the goal was to administer treatment for 1 year, the average time on treatment was 22 weeks with nivolumab monotherapy and 22 weeks with placebo. The median time on treatment with the combination therapy was just 6.4 weeks, which is also reflected in the number of infusions given, which averaged two. “With nivolumab/ipilimumab, about 50% of patients terminated treatment within the first weeks because of toxicity,” he added.”
Good Outcomes Despite Limited Treatment
Despite such limited drug exposure, the combination therapy yielded a 2-year recurrence-free survival rate of 70%, compared with 42% with nivolumab and 14% with placebo (Table 2). “I think the hazard ratio of 0.23 for nivolumab/ipilimumab vs placebo is spectacular,” Dr. Schadendorf commented. “Obviously, we also compared the combination with nivolumab, and this turned out to be statistically significant as well, with a hazard ratio of 0.40.”
Relapses occurred in 81% of the placebo arm, 56% of the nivolumab arm, and 27% of the combination arm. Distant relapses were reported in 44%, 39%, and 14%, respectively. “So, 73% of patients treated with nivolumab/ipilimumab had no relapses at all, compared with just 19% of the placebo arm,” he noted.
By subgroup, patients with BRAF-mutated tumors particularly benefited from the combination, with 87% being relapse-free (hazard ratio = 0.07 vs placebo and 0.17 vs nivolumab). The combination was strongly favored in the majority of prespecified subgroups, attaining relative risk reductions in the 70% range vs placebo.
The safety profile for nivolumab alone was superior to that of the combination, with fewer grade 3 or 4 adverse events—including fewer immune-related events, 25% vs 69%—and less toxicity leading to treatment discontinuation. The rates in both arms were higher than those reported in the CheckMate 067 trial in patients with unresectable melanoma, he added.
“These results support the use of the nivolumab/ipilimumab combination in the adjuvant treatment of advanced melanoma and await confirmation by CheckMate 915 in stage III/IV disease,” Dr. Schadendorf concluded.
CheckMate 238: Biomarker Data
Attendees at the ESMO Congress also heard the long-term (36-month) follow-up of CheckMate 238.5 In the study of 906 patients with high-risk resected stage III/IV melanoma, nivolumab reduced the risk of recurrence by 32% over ipilimumab (P < .0001), with continued benefit seen across most subgroups.
The median recurrence-free survival was not reached with nivolumab and was 24.9 months with ipilimumab (hazard ratio = 0.68; P < .0001). At 3 years, 58% and 45%, respectively, were free of recurrence; differences between the arms have widened each year, according to Jeffrey Weber, MD, of NYU Langone Medical Center, New York.

Jeffrey Weber, MD
By stage, recurrence-free survival rates were 60% with nivolumab vs 46% with ipilimumab in the stage III resected subset and 54% and 40%, respectively, among patients with stage IV. The median distant metastasis–free survival was not reached in either arm, although nivolumab was favored (hazard ratio = 0.74; P = .044).
In addition, Dr. Weber presented new biomarker analyses, which showed that—for both the drugs—treatment benefit was associated with high tumor mutational burden, high levels of tumor inflammation (interferon-γ [IFN-γ]), and low levels of peripheral suppressive immune cells (myeloid-derived suppressor cells [MDSCs]). These biomarkers were particularly strong when used together, as shown by the hazard ratios (with 95% confidence intervals) for the nivolumab cohort compared with the group that has both biomarkers low:
- High tumor mutational burden + high IFN-γ: 0.26 (0.11–0.62)
- Low MDSCs + high IFN-γ: 0.20 (0.08–0.51)
- High IFN-γ + high MDSCs: 0.35 (0.16–0.74).
If these biomarkers can be validated, it may be possible to identify patients at baseline who can be expected to obtain the most benefit from these drugs, commented Dr. Weber.
Anti–PD-1 Brain Collaboration
Nivolumab plus ipilimumab also improved intracranial response rates and survival, over nivolumab alone, in the investigator-driven ABC trial, as reported by Georgina Long, MD, PhD, of the Melanoma Institute Australia, The University of Sydney, and Mater and Royal North Shore Hospitals, Sydney.3 The ABC trial enrolled 60 patients with asymptomatic brain metastases and no prior immunotherapy to receive the combination (n = 35) or nivolumab alone (n = 25).

Georgina Long, MD, PhD
Intracranial responses were observed in 51% of the combination arm (26% complete responses) compared with 20% (16% complete responses) in the nivolumab arm; the median duration of response was not reached in either arm. The response rate rose to 59% among patients who were naive to a BRAF inhibitor plus a MEK inhibitor. Intracranial responses were concordant with extracranial responses; extracranial responses occurred in 57% of patients treated with nivolumab/ipilimu-mab vs 29% on nivolumab monotherapy, Dr. Long reported.
Intracranial progression-free survival mirrored intracranial responses, with rates of 43% at 3 years with the combination and 15% with nivolumab; the rates were 48% and 14%, respectively, in the drug-naive subset.
“With longer follow-up, upfront ipilimumab plus nivolu-mab demonstrates durable intracranial responses, with no new adverse events,” stated Dr. Long. “A study of upfront ipilimumab plus nivolumab plus stereotactic radiotherapy vs ipilimumab plus nivolumab is planned.” ■
DISCLOSURE: CheckMate 067 was funded by Bristol-Myers Squibb. Dr. Larkin has received honoraria/research funding from or served as an advisor or consultant to Achilles, AstraZeneca, Boston Biomedical, Bristol-Myers Squibb, Eisai, EUSA Pharma, GSK, Ipsen, Imugene, Incyte, Onctura, Kymab, Merck Serono, MSD, Nektar, Novartis, Pierre Fabre, Pfizer, Roche, Secarna, Aveo, Covance, and Vitaccess. Dr. Schadendorf has received honoria from or served as an advisor or consultant to Pierre Fabre, Amgen, Boehringer Ingelheim, Leo Pharma, Roche, MSD, Incyte, Regeneron, 4SC, AstraZeneca, Bristol-Myers Squibb, Merck-EMD, Pfizer, Philogen, and Array. Dr. Weber has served as a consultant to Merck, Genentech, AstraZeneca, GSK, Novartis, Nektar, Medivation, Celldex, Incyte, and EMO Serono; has served as an advisor to Bristol-Myers Squibb, Celldex, CytomX, Incyte, Biond, Protean, CV6, and Sellas; holds equity in CtoX, Biond, and Altor; and has participated in several relevant patent filings. Dr. Long has served as an advisor or consultant to Aduro, Amgen, Bristol-Myers Squibb, Mass-Array, Merck, MSD, Novartis, OncoSec Medical, Pierre Fabre, and Roche.
REFERENCES
1. Larkin JMG, Chiarion-Sileni V, Gonzalez R, et al: Five-year survival outcomes of the CheckMate 067 phase III trial of nivolumab plus ipilimumab combination therapy in advanced melanoma. ESMO Congress 2019. Abstract LBA68_PR. Presented September 28, 2019.
2. Schadendorf D, Hassel JC, Fluck M, et al: Adjuvant immunotherapy with nivolumab alone or in combination with ipilimumab vs placebo in stage IV melanoma patients with no evidence of disease: A randomized, double-blind phase II trial (IMMUNED). ESMO Congress 2019. Abstract LBA67. Presented September 28, 2019.
3. Long GV, Atkinson VG, Sandhu SK, et al: Long-term outcomes from the randomized phase II study of nivolumab or nivolumab + ipilimumab in patients with melanoma brain metastases: Anti-PD1 Brain Collaboration (ABC). ESMO Congress 2019. Abstract 1311O. Presented September 28, 2019.
4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al: Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019.
5. Weber J, Del Vecchio M, Mandala M, et al: Adjuvant nivolumab versus ipilimumab in resected stage III/IV melanoma: 3-year efficacy and biomarker results from the phase III CheckMate 238 trial. ESMO Congress 2019. Abstract 2801. Presented September 28, 2019.

