
Barry Paul, MD

Saad Z. Usmani, MD, FACP
The term “relapsed/refractory multiple myeloma” is often used to describe advanced myeloma that has progressed through primary or salvage therapy. The International Myeloma Working Group (IMWG) defined the term in 2011 as disease that is “nonresponsive while on salvage therapy or progresses within 60 days of last therapy in patients who have achieved minimal response or better at some point previously before then progressing in their disease course.”1
In the 8 years since this definition was published, seven new agents or combinations have been approved for the treatment of multiple myeloma, and several other therapies are currently in late-stage or registrational trials, with possible approvals in the near future.2 The influx of these newer agents has significantly improved the prognosis of patients with myeloma, especially those with disease that has become resistant to conventional therapies.
The most recent addition to the list of U.S. Food and Drug Administration (FDA)-approved myeloma therapies is the exportin 1 (XPO1) inhibitor selinexor, which was approved in July 2019 based on the data obtained in part 2 of the phase IIb STORM trial—reported by Chari et al3 and reviewed in this issue of The ASCO Post. The STORM trial evaluated the combination of selinexor and dexamethasone in a heavily pretreated population. Specifically, inclusion criteria required previous treatment with bortezomib, carfilzomib, lenalidomide, pomalidomide, daratumumab, corticosteroids, and an alkylating agent. Additionally, patients needed to be defined as refractory to at least one immunomodulatory agent, one proteasome inhibitor, daratumumab, and glucocorticoids.
STORM Patient Population
The trial enrolled 123 patients between May 2015 and March 2018 at 60 sites in the United States and Europe. Patients were treated with selinexor at 80 mg and dexamethasone at 20 mg twice weekly (days 1 and 3) in 4-week cycles until disease progression, death, or intolerance. The primary endpoint was overall response (defined as a partial response or better by IMWG criteria). Secondary endpoints included the duration of response, clinical benefit rate (defined as ≥ 25% decrease in serum myeloma markers), progression-free survival, and overall survival.
Reviewing the baseline characteristics of the enrolled population confirms their severe refractoriness. The median time since initial diagnosis was 6.6 years, and 53% of patients had high-risk cytogenetics (defined as the presence of del 17p, t[4;14], t[14;16], or more than two copies of 1q21). Additionally, 68% of the patients were so-called penta-refractory—refractory to bortezomib, carfilzomib, lenalidomide, pomalidomide, and daratumumab.
STORM Efficacy Outcomes and Safety
At the time of data cutoff (August 17, 2018), 118 patients (96%) had discontinued treatment, and the median duration of treatment was 9 weeks. The most common indications for treatment discontinuation were disease progression (55%) and adverse events (31%). A partial response or better was seen in 32 patients (26%), with 2 (2%) complete responses, 6 (5%) very good partial responses, and 24 (20%) partial responses. Additionally, another 16 patients (13%) achieved a minor response, and 48 patients (39%) had confirmed stable disease. Taken together, the disease control rate (confirmed minimal response or better) was 39%.
“The response rates in these trials in patients with relapsed/refractory multiple myeloma appear inversely proportional to the number of prior lines of therapies.”— Barry Paul, MD, and Saad Z. Usmani, MD, FACP
Tweet this quote
Common toxicities associated with selinexor/dexamethasone treatment were thrombocytopenia (73%), fatigue (73%), nausea (72%), and anemia (67%). The most common grade 3 or 4 toxicities were thrombocytopenia (59%), anemia (44%), hyponatremia (22%), and neutropenia (21%). Of note, six patients (5%) with grade 3 or 4 thrombocytopenia had a grade 3 or higher bleeding event.
Dose modifications or interruptions were required in 80% of patients, most commonly due to thrombocytopenia. Serious adverse events occurred in 77 patients (63%), and a total of 38 patients (31%) discontinued treatment due to adverse events. The investigators concluded that 23 patients discontinued treatment due to treatment-related adverse events, and an additional 15 patients discontinued therapy due to an adverse event “unrelated” to selinexor/dexamethasone. The investigators did recognize the difficulty in assigning causality with such a new combination.
Partly Sunny or Mostly Cloudy?
Overall, the response rate was admittedly modest, and the toxicities were significant, which has led to valid discussions about the risk/benefit ratio of this combination in patients with multiple myeloma. Indeed, an FDA advisory panel originally voted 8 to 5 to delay the accelerated approval of the combination earlier in 2019, based in large part on the high rates of serious adverse events. However, when the combination’s role in multiple myeloma therapy is assessed, it is important to interpret these data in the context of the population treated. This is especially pertinent when recognizing that these response rates are maintained in the penta-refractory population.
Given that many of the agents that patients with penta-refractory disease have acquired resistance to have themselves only recently been approved, one can assume that penta-refractory multiple myeloma represents a novel phenotype that differs from traditional relapsed/refractory multiple myeloma and underscores the importance of defining the “refractoriness” of patients when interpreting data in trials in this population. This is further supported by noting a median 22% increase in serologic myeloma markers in the 107 patients, with data obtained on both screening and on day 1 of treatment (median duration of 12 days), suggesting highly proliferative disease at the time of the first dose.
It is important to appreciate what “relapsed/refractory” populations were enrolled in each of the trials leading to FDA approval of single drugs or combinations (see Table 1). For example, none of the patients in the SUMMIT trial were refractory to a “proteasome inhibitor,” because bortezomib was a first-in-class agent.
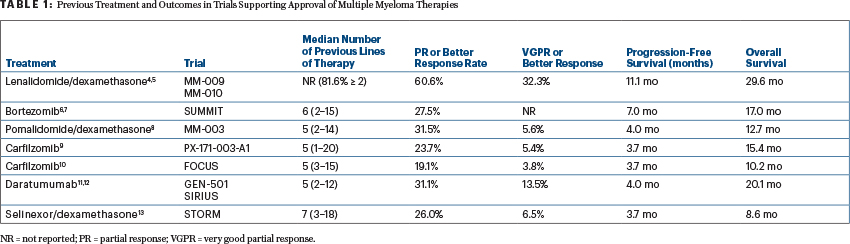
With this context in mind, the response rates in these trials of patients with relapsed/refractory multiple myeloma appear inversely proportional to the number of prior lines of therapies.4-12 The overall response rate (partial response or better in 26%) and progression-free survival (median of 3.7 months) with selinexor/dexamethasone compare favorably with those seen in the MM-003 trial of pomalidomide/dexamethasone (response rate of 31.5%; median progression-free survival of 4 months), the PX-171-003-A1 trial of carfilzomib (23.7%; 3.7 months), and the GEN-501 and SIRIUS trials of single-agent daratumumab (31.1%; 4 months)—and in all of these trials, patients had a median of five or more prior lines of therapy.3,8,9,11 However, the combination of selinexor and dexamethasone did have a significantly higher rate of adverse events, which is particularly concerning, since the STORM investigators classified 39% of the adverse events requiring discontinuation as “unrelated” to the treatment. Given the novelty of this combination and the relatively limited safety data, this may represent an underestimation of the true toxicity profile.
This question may be addressed by the phase III BOSTON trial, which is currently enrolling a less-refractory patient population (one to three prior lines of therapy) and is employing a different dose of selinexor (100 mg on days 1, 8, 15, 22, and 29 of each 35-day cycle) in combination with bortezomib and dexamethasone. Previous data from a phase I/II trial with this combination reported significantly lower rates of grade 3 or 4 adverse events than those reported in the STORM trial, even with the addition of a third agent.13 However, is the BOSTON trial a confirmatory study for the STORM trial? The answer is “no”—the study populations are very different, and both trials are asking very different questions.
Proceed With Caution
Taken together, the data obtained from the STORM trial show that XPO1 inhibition provides clinical benefit in patients with highly relapsed/refractory multiple myeloma, a population representing a significant unmet medical need and one likely to grow in the future. Additionally, selinexor’s novel mechanism of action has the potential to complement several other lines of therapy that currently represent the standard of care in multiple myeloma treatment. However, the optimal dosing strategy and combination partners to maximize benefit and minimize toxicity remain to be elucidated. Until such data are available, providers should proceed cautiously with the use of this combination and must weigh the specific risks and benefits with each individual patient prior to initiating therapy.
Dr. Paul practices in the Division of Plasma Cell Disorders, Department of Hematologic Oncology and Blood Disorders, Levine Cancer Institute, Carolinas HealthCare System, Charlotte, North Carolina. Dr. Usmani is Chief, Plasma Cell Disorders and Director, Clinical Research in Hematologic Malignancies, Department of Hematologic Oncology and Blood Disorders, Levine Cancer Institute, Carolinas HealthCare System, Charlotte.
DISCLOSURE: Dr. Paul owns stock or other ownership interests in Bristol-Myers Squibb. Dr. Usmani has served as a consultant or advisor to AbbVie, Amgen, Celgene, GlaxoSmithKline, Janssen Oncology, Karyopharm Therapeutics, Seattle Genetics, SkylineDX, and Takeda; has served on a speakers bureau for Amgen, Celgene, Janssen Oncology, and Takeda; and has received research funding from Amgen, Array BioPharma, Bristol-Myers Squibb, Celgene, Janssen Oncology, Pharmacyclics, Seattle Genetics, and Sanofi.
REFERENCES
1. Rajkumar SV, Harousseau JL, Durie B, et al: Consensus recommendations for the uniform reporting of clinical trials: Report of the International Myeloma Workshop Consensus Panel 1. Blood 117:4691-4695, 2011.
2. Anderson KC: Progress and paradigms in multiple myeloma. Clin Cancer Res 22:5419-5427, 2016.
3. Chari A, Vogl DT, Gavriatopoulou M, et al: Oral selinexor-dexamethasone for triple-class refractory multiple myeloma. N Engl J Med 381:727-738, 2019.
4. Weber DM, Chen C, Niesvizky R, et al: Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med 357:2133-2142, 2007.
5. Dimopoulos MA, Chen C, Spencer A, et al: Long-term follow-up on overall survival from the MM-009 and MM-010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia 23:2147-2152, 2009.
6. Richardson PG, Barlogie B, Berenson J, et al: A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609-2617, 2003.
7. Richardson PG, Barlogie B, Berenson J, et al: Extended follow-up of a phase II trial in relapsed, refractory multiple myeloma: Final time-to-event results from the SUMMIT trial. Cancer 106:1316-1319, 2006.
8. Miguel JS, Weisel K, Moreau P, et al: Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): A randomised, open-label, phase 3 trial. Lancet Oncol 14:1055-1066, 2013.
9. Siegel DS, Martin T, Wang M, et al: A phase 2 study of single-agent carfilzomib (PX-171-003-A1) in patients with relapsed and refractory multiple myeloma. Blood 120:2817-2825, 2012.
10. Hájek R, Masszi T, Petrucci MT, et al: A randomized phase III study of carfilzomib vs low-dose corticosteroids with optional cyclophosphamide in relapsed and refractory multiple myeloma (FOCUS). Leukemia 31:107-114, 2017.
11. Usmani SZ, Weiss BM, Plesner T, et al: Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 128:37-44, 2016.
12. Lonial S, Weiss BM, Usmani SZ, et al: Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387:1551-1560, 2016.
13. Bahlis NJ, Sutherland H, White D, et al: Selinexor plus low-dose bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma. Blood 132:2546-2554, 2018.

