In the first-line treatment of advanced hepatocellular carcinoma, checkpoint inhibitors led to favorable outcomes in studies reported at the European Society for Medical Oncology (ESMO) Congress 2019—though one study was technically negative.
The current first-line standard of care for unresectable or metastatic hepatocellular carcinoma is treatment with a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor; these drugs offer modest survival benefits and considerable toxicity. Single-agent checkpoint inhibitors have shown activity but have not yet demonstrated superiority in randomized trials.

The encouraging efficacy and favorable safety profile seen with nivolumab demonstrate the potential benefit of immunotherapy as a first-line treatment for patients with [hepatocellular carcinoma].— Thomas Yau, MBBS, MD
Tweet this quote
The phase III CheckMate 459 trial compared nivolumab to sorafenib as first-line treatment in 743 patients with advanced hepatocellular carcinoma.1 The threshold for a statistically significant improvement in overall survival was not met. However, presenting author Thomas Yau, MBBS, MD, of The University of Hong Kong, maintained, “The primary analysis demonstrated a clinically meaningful overall survival benefit, which is particularly impactful considering the high frequency of subsequent use of systemic therapy, including immunotherapy in the sorafenib arm. Importantly, there was also a higher complete response rate with nivolumab compared to sorafenib.”
Michael S. Lee, MD, of the University of North Carolina Lineberger Comprehensive Cancer Center in Chapel Hill, presented the phase 1b GO30140 study of atezolizumab plus bevacizumab, showing “clinically meaningful and durable responses” and “superiority” of the combination over atezolizumab monotherapy.2
CheckMate 459
The phase III CheckMate 459 study enrolled 743 patients with advanced hepatocellular carcinoma who were not eligible for, or whose disease progressed after, resection or radiotherapy. Patients were randomly assigned to nivolumab at 240 mg every 2 weeks or sorafenib at 400 mg twice daily as first-line therapy.
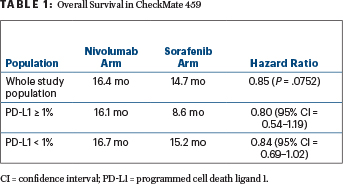
Median overall survival was improved with nivolumab (hazard ratio [HR] = 0.85; P = .0752), though statistical significance was not met (Table 1). One factor could have been subsequent systemic therapy received by 46% in the sorafenib arm (20% immunotherapy), investigators suggested.
Median progression-free survival was 3.7 months and 3.8 months, respectively (HR = 0.93, 95% confidence interval [CI] = 0.79–1.10), though numeric improvements were seen with nivolumab at 12 months (22% progression-free vs 14% in the sorafenib arm) and at 24 months (14% vs 6%, respectively). The Kaplan-Meier curves begin to separate at 6 months, Dr. Yau noted.

Atezolizumab plus bevacizumab may become a promising treatment option for patients with unresectable hepatocellular carcinoma.— Michael S. Lee, MD
Tweet this quote
Clinical benefit was observed across subgroups, including patients with hepatitis infection (HR = 0.71–0.77), vascular invasion and/or extrahepatic spread (HR = 0.74), and programmed cell death ligand 1 (PD-L1) expression ≥ 1% (HR = 0.80) and < 1% (HR = 0.84),“though there was a trend toward better overall survival and response in patients with PD-L1 ≥ 1%,” he said (Table 1). Asian patients, patients < 65 years old, and patients with higher alpha-fetoprotein levels were also among the subgroups who had the greatest benefit.
The overall response rate was 15% for nivolumab (including 14 patients with a complete response) and 7% for sorafenib (5 patients with a complete response). Grade 3 or 4 treatment-related adverse were reported in 22% of patients on nivolumab and 49% on sorafenib, leading to treatment discontinuation in 4% and 8%, respectively. Clinically meaningful differences were observed in health-related quality of life favoring nivolumab, with less worsening of treatment-related side effects reported.
“These results are important in the treatment of hepatocellular carcinoma, as there have been no significant advances over sorafenib in the first-line setting in more than a decade,” said Dr. Yau.“The encouraging efficacy and favorable safety profile seen with nivolumab demonstrate the potential benefit of immunotherapy as a first-line treatment for patients with this aggressive cancer.”
Atezolizumab/Bevacizumab
There is strong rationale for combining an anti–programmed cell death protein 1 antibody and the VEGFR inhibitor bevacizumab, since many hepatocellular carcinomas are hypervascular and overexpress VEGF and PD-L1, according to Dr. Lee. “VEGF inhibition may help reverse VEGF-mediated immunosuppression and enhance anticancer immunity, and dual blockade of PD-L1 and VEGF has shown clinical benefit in other tumor types,” he said.
To evaluate the benefit of this approach in hepatocellular carcinoma, the GO30140 study randomly assigned patients with advanced disease who had had no prior systemic therapy with atezolizumab/bevacizumab or atezolizumab monotherapy. The design had two main arms with different endpoints:
Arm A: every-3-week cycles of atezolizumab (1,200 mg) plus bevacizumab (15 mg); primary endpoints were objective response rate by independent review and safety (n = 104). Median follow-up was 12.4 months.
Arm F: every-3-week cycles of atezolizumab at 1,200 mg plus bevacizumab at 15 mg (n = 60) OR atezolizumab monotherapy at 1,200 mg (n = 59); primary endpoints were progression-free survival by independent review and safety. Median follow-up was 6.6 months.
Outcomes in Each Arm
In arm A, atezolizumab/bevacizumab led to a response rate of 36%, with 12% complete responses, which Dr. Lee called “impressive.” Three-quarters of responses were ongoing at data cutoff, with median durations of response not reached. Median progression-free survival was 7.3 months, and median overall survival was 17.1 months. At 12 months, 63% of patients were still alive.
In arm F, median progression-free survival was 5.6 months with atezolizumab/bevacizumab vs 3.4 months with atezolizumab (HR = 0.55; P = .0108). Responses were confirmed in 20% vs 17%, respectively, with median durations of response not reached in either arm at 6.6 months’ follow-up. The disease control rate was 67% and 49%, respectively.
IMMUNOTHERAPY IN HEPATOCELLULAR CARCINOMA
- In patients with advanced hepatocellular carcinoma, median overall survival was 16.4 months with nivolumab and 14.7 months with sorafenib, showing a trend for improvement that did not meet the predefined threshold of statistical significance in the phase III CheckMate 459 trial.
- The overall response rate was 15% for nivolumab and 7% for sorafenib.
- Nivolumab had a favorable safety profile, with fewer toxicity-related treatment discontinuations compared to sorafenib.
- The combination of atezolizumab and bevacizumab led to high response rates and a 45% reduction in the risk of disease progression in a phase1b study.
“Overall, the combination was well tolerated,” Dr. Lee said. Grade 3 or 4 treatment-related adverse events occurred in 20% on the combination vs 5%, but there were no grade 5 events, and only 3% and 2% per arm discontinued treatment because of them. “Adverse events of special interest, due to the known toxicities of the drugs, were comparable…. Although hypertension was more common with the combination, the rate of grade 3 or 4 hypertension was low (5%) with the combination and also with atezolizumab alone (2%)…. We had reassuringly low rates of gastrointestinal bleeding (3% and 2%, respectively).”
Dr. Lee maintained, “Atezolizumab plus bevacizumab may become a promising treatment option for patients with unresectable hepatocellular carcinoma.” This combination is being evaluated in the phase III IMbrave150 trial. ■
DISCLOSURE: CheckMate 459 was funded by Bristol-Myers Squibb. Dr. Yau reported financial relationships with Bristol-Myers Squibb. The atezolizumab/bevacizumab study was sponsored by F. Hoffmann-LaRoche. Dr. Lee has consulted or advised for AstraZeneca.
REFERENCES
1. Yau T, Park JW, Finn RS, et al: CheckMate 459: A randomized, multicenter phase 3 study of nivolumab vs sorafenib as first-line treatment in patients with advanced hepatocellular carcinoma. ESMO Congress 2019. Abstract LBA38_PR. Presented September 27, 2019.
2. Lee MS, Ryoo B-Y, Hsu C-H, et al: Randomized efficacy and safety results for atezolizumab + bevacizumab in patients with previously untreated, unresectable hepatocellular carcinoma. ESMO Congress 2019. Abstract LBA39. Presented September 27, 2019.


