With the availability of at least five checkpoint inhibitors to treat non–small cell lung cancer (NSCLC) and other solid tumors, appropriate patient selection for these expensive treatments remains key. The hope is that testing the level of programmed cell death ligand 1 (PD-L1) expression in tumor cells could identify patients most likely to respond to checkpoint inhibitor therapy.
The current situation is “chaotic,” according to some experts. Each of the five checkpoint inhibitors has its own diagnostic assay relying on immunohistochemistry (IHC) staining of tumor cells, and in one case, immune cells as well. The five tests differ in key ways: antibody-binding epitopes, strainers, detection systems, and protocols.

We found that the three PD-L1 tests were interchangeable as long as the clinical algorithm matched with the individual drug in patient selection.— Ming S. Tsao, MD
Tweet this quote
A current global effort is seeking to determine the best use of these PD-L1 assays. Of five available PD-L1 assays, three appear to be interchangeable (28-8, 22C3, SP263), according to the results of the Blueprint phase IIA study presented by Ming S. Tsao, MD, of Princess Margaret Cancer Centre, Toronto, at the recent International Association for the Study of Lung Cancer (IASLC) 18th World Conference on Lung Cancer.1 The phase IIA study also found that staining of tumor cells is more reliable than staining of immune cells.
The fact that three of the tests appear to be interchangeable opens the door for using one test when considering treatment with any of the three drugs in question. At present, however, this remains a research question, and more studies are needed to confirm whether labs can extrapolate results from one PD-L1 assay to another.
“The Blueprint Project results indicate that 28-8, 22C3, and SP263 perform very similarly regarding PD-L1 expression on tumor cells. They might be interchangeable in the clinical setting, but we do not have any clinical data yet to verify that. We have only analytic data,” said IASLC Chief Executive Officer Fred R. Hirsch, MD, Professor of Medicine and Pathology, University of Colorado Cancer Center, Denver, and study co-investigator.
Background and Study Details
“It is challenging for labs to offer all these biomarker assays. We have questions, such as how different are these tests from one another, and are they interchangeable as far as predictive value goes?” explained Dr. Tsao.
The Blueprint Project is a global effort aimed at answering these questions. In Blueprint phase I, four different assays were studied: 28-8, 22C3, SP263, and SP142.2 Three of these assays—28-8, 22C3, SP263—were found to be comparable with high levels of concordance for assessment of PD-L1 staining on tumor cells, whereas SP142 was an outlier, or “unique,” as Dr. Tsao said. The phase I findings were based on studies of paraffin blocks of 39 resected NSCLC tumor samples interpreted by three pathologists.

Perhaps a combination of biomarkers—for example, PD-L1 expression and high tumor mutation burden—will turn out to be a better predictive indicator.— Fred R. Hirsch, MD
Tweet this quote
Phase IIA compared the reliability of the four tests from phase I and a fifth IHC test, 73-10, using a panel of 25 pathologists (1 trainer) to evaluate 81 “real-life” tumor samples from NSCLC patients being treated in routine clinical practice. Samples were from resection, lymph node biopsy, core bronchial biopsy, and cytology cell block and were scored without awareness of the tissue source or type of sample and randomized.
“Our findings confirmed the phase I findings, ie, that the three assays were comparable. SP142 had less staining of PD-L1–positive tumor cells, whereas 73-10 had more staining,” Dr. Tsao said.
For all cases, scoring immune cell staining had poor reliability and was more challenging, with poor concordance among pathologists. Digital scoring was as reliable as glass slides, he noted.
“In the limited number of cytology cases [22 samples], only moderate reliability was seen compared with high reliability on tissue scoring. We hope to get more data on cytology to make definitive conclusions,” Dr. Tsao said.
“We found that the three PD-L1 tests were interchangeable as long as the clinical algorithm matched with the individual drug in patient selection.… We need more data on 73-10,” he said.
Who Should Undergo PD-L1 Testing?
“At this time, the cutoffs for PD-L1 positivity vary for the different drugs and in clinical trials. The only cutoff that is U.S. Food and Drug Administration (FDA)-approved for lung cancer is for pembrolizumab. For first-line therapy of NSCLC, the cutoff is a tumor proportion score of 50% or higher,” said Dr. Hirsch.
A recent study suggested that stringent use of PD-L1 assays for all NSCLC patients could exclude 28% of patients who may benefit from immune checkpoint inhibitor therapy and include up to 42% of patients who may have no benefit.3
“Although PD-L1 is an imperfect biomarker, several studies have shown some predictive capability for PD-L1 testing. In general, patients with high PD-L1 expression have better outcomes than those with low or no expression, but there are patients who also benefit with low or no PD-L1 expression. Perhaps a combination of biomarkers—for example, PD-L1 expression and high tumor mutation burden—will turn out to be a better predictive indicator,” Dr. Hirsch continued.
Currently, in the clinical setting outside of trials, PD-L1 testing is advised for those with advanced NSCLC who do not have tumors harboring epidermal growth factor receptor (EGFR) mutation or rearrangements in the ALK or ROS gene and who are being considered for pembrolizumab therapy.
PD-L1 IHC ASSAYS
- Three of five appear to be interchangeable.
- Thus, it might be possible to use only one assay when considering any of the three drugs for which the tests were developed.
- In the clinical setting, PD-L1 testing is currently required for pembrolizumab treatment selection in NSCLC.
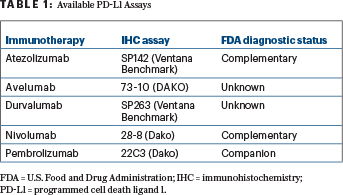
“For first-line therapy of NSCLC, the pembrolizumab 22C3 assay is FDA-approved with a tumor proportion score of 50% or more as the cutoff. Otherwise, it is still an ongoing research tool in clinical trials. For second-line therapy of NSCLC, pembrolizumab is FDA-approved with the companion diagnostic test using a lower cutoff (ie, > 1%), and other immunotherapy agents are approved with PD-L1 as a complementary diagnostic,” Dr. Hirsch said. A companion test is essential for use of the drug, whereas a complementary assay is used to optimize patient selection, he clarified (see Table 1 on page 28). ■
DISCLOSURE: Dr. Tsao has received consultancy honoraria for from AstraZeneca, BMS, Merck and Ventana/Hoffmann La Roche and a research grant from Merck. Dr. Hirsch reported no conflicts of interest.
REFERENCES
1. Tsao MS, Yatabe Y, Hirsch FR, et al: Blueprint 2: PD-L1 immunohistochemistry comparability in real-life clinical samples. 2017 IASLC World Conference on Lung Cancer. Abstract PL3.03. Presented October 18, 2017.
2. Hirsch FR, McElhinny A, Stanforth D, et al: PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the Blueprint PD-L1 IHC Assay Comparison Project. J Thorac Oncol 2:208-222, 2017.
3. Diggs LP, Hsueh EC: Utlility of PD-L1 immunohistochemistry assays for predicting PD-1/PD-L1 inhibitor response. Biomark Res 5:12, 2017.

