Trials with pazopanib (Votrient) have “provided significant efficacy, toxicity, and tolerability data for pazopanib to be established as a first-line standard of care” for renal cell carcinoma,” Tim Eisen, PhD, of the University of Cambridge, United Kingdom, stated at the 11th International Kidney Cancer Symposium in Chicago. He noted that the pazopanib trials show noninferiority relative to sunitinib (Sutent) for progression-free survival, although the “safety profile favors pazopanib,” with the exception of liver function test abnormalities.
“In the future, tivozanib may be a first-line standard of care, but for me, this is a premature conclusion right now. We do need comparative data with sunitinib, pazopanib, or axitinib [Inlyta],” Dr. Eisen added. Moving axitinib into front-line treatment for renal cell carcinoma also depends on data from phase III trials. In addition, “there is no way yet of targeting therapy, and that is something we continue to work on,” Dr. Eisen said.
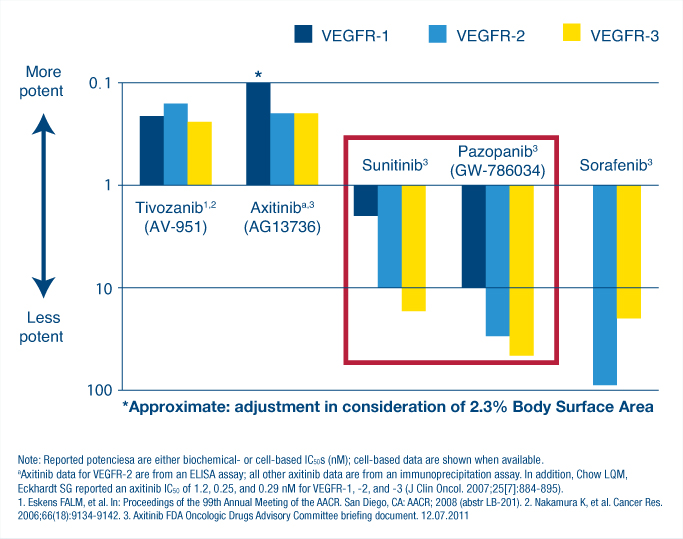
Dr. Eisen’s conclusions followed presentations on recent trials of tyrosine kinase inhibitors of vascular endothelial growth factor (VEGF)-1, -2, and -3. used to treat renal cell carcinoma. “First-generation agents include pazopanib, sunitinib, and sorafenib [Nexavar],” Dr. Eisen said. The second-generation agents, which he listed as tivozanib and axitinib, “are both more specific in targeting VEGF receptors preferentially and also more potent,” he added (see Fig. 1).
“Kidney cancer is fundamentally a VEGF-driven disease,” noted Brian R. Rini, MD, of the Department of Solid Tumor Oncology, Cleveland Clinic Taussig Cancer Institute, and Glickman Urologic and Kidney Institute. “It follows that precise and potent inhibition of VEGF will produce the most robust clinical effects, and that this really should be applied as early as possible in the course of treatment before other alternative pathways have a chance to emerge.”
Axitinib Trials
Axitinib “is the most biochemically potent agent and selective second-generation inhibitor of the VEGF family of receptors, [with] demonstrated efficacy as second-line treatment for metastatic renal cell carcinoma,” Dr. Rini said. In the phase II 1046 trial (reported at this year’s ASCO Annual Meeting1), axitinib was shown to produce a median progression-free survival of 14.5 months in the entire cohort of 213 patients with metastatic disease with clear cell histology and a median progression-free survival of 16.4 months in a subset of 91 patients who did not meet criteria for dose titration, mainly due to hypertension. “These patients had the best outcomes, not surprisingly, because they had adequate drug levels. Overall response rates were 48% in the total cohort and 59% in the subset.
“If a patient doesn’t meet criteria for titration, he will likely have adequate levels. This isn’t true for every single patient, but for a group of patients, it is going to be true. In contrast, patients who are eligible for titration generally have inadequate levels,” Dr. Rini explained. “So titration upward is not to achieve super-high drug levels…. It is to achieve adequate drug levels and give patients a chance to respond.” Patients who did meet criteria for titration were either titrated stepwise to 7 mg twice daily (to a maximum of 10 mg twice daily) or received placebo dose titration (blinded therapy).
Hypertension Connection
Hypertension is not only associated with higher drug levels and advantages in progression-free survival and response rate, but “is the dominant adverse event,” Dr. Rini said. Hypertension of all grades occurred in 135 patients (63%) and of grade 3/4 in 61 patients (29%). “The numbers are higher than have been reported for axitinib previously, mostly because there was more intensive blood pressure monitoring and there was also more aggressive dose titration in this trial.” Dr. Rini said. Other common adverse events included diarrhea (all grades occurring in 58% of patients and grades 3/4 in 7%), fatigue (all grades occurring in 48% of patients and grades 3/4 in 6%), and dysphonia (all grades occurring in 40% of patients, but grades 3/4 in only 1%).
“It is my belief that axitinib is the most effective first-line treatment for metastatic renal cell carcinoma, as evidenced by a very long progression-free survival and a high objective response rate based on preliminary data,” Dr. Rini concluded. “When optimally titrated, axitinib can produce a progression-free survival of over 16 months and a response rate of nearly 60%” in previously untreated clear cell renal cell carcinoma, he continued. “I think many other oral drugs, including the tyrosine kinase inhibitors we use in kidney cancer, are being underdosed in many patients and probably not being optimally used, resulting in suboptimal efficacy,” he said.
“We are waiting for the phase III trial to be ready soon, which is a front-line trial of axitinib compared to sorafenib,” Dr. Rini said. “The primary endpoint is progression-free survival and along with the unblinding of the 1046 data, it will provide a more precise and more robust estimate of the activity of axitinib in the front-line setting, which I believe is substantial.”
The Case for Panozanib
Taken together, the results of the PISCES2 and COMPARZ3 trials make a strong case for the use of panozanib as first-line treatment for advanced renal cell carcinoma, according to Camillo Porta, MD, of the Istituto di Ricovero e Cura a Carattere Scientifico, San Matteo University Hospital Foundation, Pavia, Italy. He cautioned, however, that “we should wait for the final publication of the two studies to really go deeply through the data.”
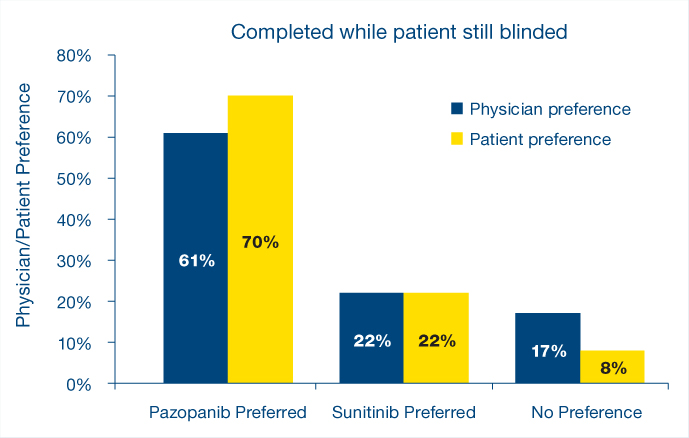
The double-blind PISCES study evaluated patient preference of two different sequences of treatment: pazopanib given for 10 weeks and then after a 2-week washout, sunitinib for 10 more weeks, or the reverse sequence. Both drugs were given at the standard doses and schedules, 800 mg once daily for pazopanib, and for sunitinib, 50 mg once daily, 4 weeks on treatment, followed by 2 weeks matching placebo, then 4 weeks on treatment.
“Significantly more patients preferred pazopanib vs sunitinib due to better general quality of life and less fatigue,” Dr. Porta said. “There was a consistency between patient and physician preference and health-related quality of life that statistically favored pazopanib for fatigue, foot/hand soreness, and mouth/throat soreness. Furthermore, fewer dose modifications were observed in patients taking pazopanib,” he said.
“The PISCES study is actually very important,” Dr. Eisen noted, because patient preference cannot easily be measured. “This is an abstract concept, and the patient that has been on both agents is in a hugely better position than a patient that has been on one agent and has nothing with which to compare that experience,” he continued. “The number of patients who were not able to express a preference was less than 10%. For me, this is convincing” (see Fig. 2).
“This innovative trial clearly demonstrated the better tolerability of pazopanib over sunitinib, despite the more modest difference in reported adverse events,” Dr. Porta stated. “This study design does not allow an efficacy comparison, but a comparison between the two drugs has just been reported, with the presentation of the results of the phase III COMPARZ trial at [the 35th European Society for Medical Oncology (ESMO) Congress].”
Using the same dosing and schedule as the PISCES trial, the COMPARZ trial, reported in the November 1 issue of The ASCO Post,4 demonstrated that pazopanib is not inferior to sunitinib. “Progression-free survival was 8.4 months for pazopanib vs 9.5 months for sunitinib. The 95% confidence interval of the two progression-free survivals clearly overlap, with a hazard ratio of 1.047, meaning that the trial demonstrated pazopanib is not inferior compared to sunitinib in terms of progression-free survival,” Dr. Porta stated. Quality of life, a secondary endpoint of the study, was superior in the pazopanib arm, “thus corroborating the results of the PISCES study,” according to Dr. Porta.
Timing of Assessments
“We now have two recent analyses that examine the impact of sunitinib dosing schedule on measurement of patient-reported fatigue and health-related quality of life,” Dr. Porta said.“ He noted that presentations at the ESMO genitourinary poster discussions questioned whether “the timing of assessment of patients’ preference clearly [did not favor] sunitinib” in both the PISCES and COMPARZ trials.
“Compared with baseline, patients reported worse fatigue during the first cycle of sunitinib treatment; however, less fatigue was reported in all consecutive treatment cycles.” In addition, the sunitinib schedule “was associated with a clear-cut on/off effect,” Dr. Porta noted, with patients reporting more fatigue at the end of the 4-week-on period and less fatigue on day 1 after the 2-week rest period. “This effect should be accounted for when collecting health-related quality-of-life data,” he said.
Another relevant question raised at the ESMO genitourinary poster discussion was whether patients in the PISCES study were informed that some side effects, such as hypertension and hand-foot skin reaction, are considered predictive of efficacy and how that might have influenced patient preference. “Nobody can answer this question, but it could make a lot of difference,” Dr. Porta said.
Dr. Eisen concurred that the study assessments for the COMPARZ trial “are not entirely acceptable in that the timing definitely favored (or would be expected to definitely favor) pazopanib” for the reasons presented by Dr. Porta. “Baseline assessment and then every 28th day of every cycle assessment for quality of life certainly will have had an impact for the reasons you’ve heard. Patients on sunitinib treatment on day 28 of each cycle are feeling at their worst.”
In addition,“disease assessments were taken when patients were still on pazopanib but had been off sunitinib for 2 weeks, and there is a possibility that that would make a difference to the disease assessment and the progression-free survival assessment,” Dr. Eisen said. “It would not, in my view, make any difference at all to the overall survival assessment, and I also expect it to have less of an impact on disease-free survival for patients who got a large number of cycles,” he continued. “We are seeing 28- to 29-month overall survival. Compare that with what we were achieving 11 or 12 years ago with interferon, when it was around 8.5 months, and I think we have made significant progress.”
Adverse Effects
One of the two most important reasons for pazopanib preference by patients in the primary analyses of the PISCES study was less fatigue, Dr. Porta said. “The other most important reason was ‘no single reason,’ meaning that patients were simply not able to explain the exact reason for their choice, which is realistically multifactorial.”
Dr. Eisen characterized the adverse effects as either on target—hypertension, hypothyroidism, and dysphonia—or off target. “The off-target effects vary between agents, and they are largely due to kinase inhibitor properties other than the main ones we are interested in.” Off-target effects include fatigue, hand-foot syndrome, diarrhea, rash, liver function test changes, peripheral edema, myelosuppression, stomatitis, and hair color change. “Differences between sunitinib and pazopanib are basically off-target effects. Pazopanib did worse for liver function test abnormalities and for weight decrease. Those two things could be of importance to patients. The liver function tests are definitely of importance to the doctor who needs to assess those, but they would be of importance to patients if they [showed rapid increases], because you need to interrupt treatment and modify it. But in terms of everyday side effects, pazopanib seemed to cause less of the three things that in my practice matter most to patients—fatigue, skin toxicity, and stomatitis.”
‘New Kid on the Block’
“Tivozanib is the new kid on the block, but it is a very exciting, promising drug that I think is going to play a major role in first-line treatment of renal cell carcinoma in the next few years,” noted Robert J. Motzer, MD, of Memorial Sloan-Kettering Cancer Center in New York. “When we think about tivozanib, we should think of three words: efficacy, safety, and tolerability.”
A highly potent inhibitor of VEGF receptors 1, 2, and 3, tivozanib is also selective, with “fewer off-target kinase activities than some of the earlier developed tyrosine kinase inhibitors. It has a favorable pharmacokinetic profile, with a half-life of about 4 days, which allows a once daily dosing schedule, 3 weeks on and 1 week off,” Dr. Motzer said. He stressed that the schedule with the week off was based on the pharmacokinetic profile, “and not symptoms developing on treatment, as in the case of sunitinib. The sunitinib holiday was built in because of the intolerability around day 28. Not so with tivozanib. This was based on the pharmacokinetic profile, and patients don’t feel poorly around day 21.”
A phase II trial of tivozanib in 272 patients with advanced renal cell carcinoma “showed a high level of efficacy and good safety profile, and that led to the TIVO-1 trial, which we presented at ASCO,”5 Dr. Motzer said. This is a phase III superiority trial of tivozanib vs sorafenib as first-line targeted therapy for metastatic renal cell carcinoma. Patients could have received no or one prior therapy, but no prior VEGF or mTOR therapy. Tivozanib was given orally at 1.5 mg, in a 3-week-on, 1-week-off schedule, and sorafenib was given orally at 400 mg twice daily, continuously. “One of the unique features that was built into this study was that patients who were on sorafenib, at time of progression, were offered tivozanib on a second separate extension protocol,” Dr. Motzer said.
Impact of Subsequent Treatment
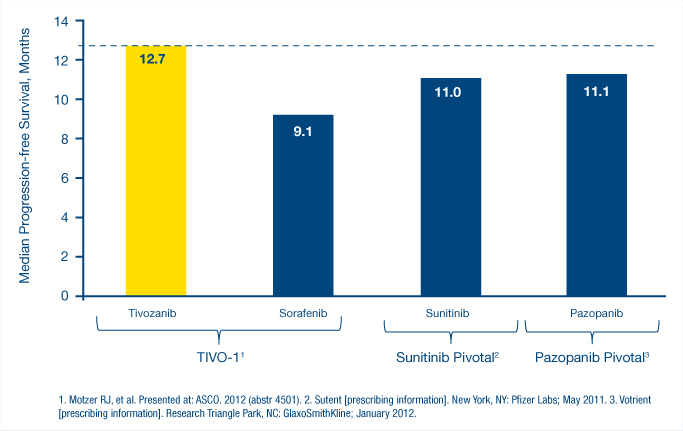
“The efficacy of tivozanib was demonstrated both in the overall population as well as the treatment-naive population, with those patients receiving tivozanib as first-line therapy. And all patients receive it as the first-line targeted therapy.” Cautioning that “this is a work in progress,” Dr. Motzer noted that the study showed median progression-free survival rates, according to an independent review, of 12.7 months for tivozanib vs 9.1 months for sorafenib. “The median progression-free survival for currently approved treatment-naive therapies “has been 11 months or less, so certainly there is room for improvement,” Dr. Motzer said (see Fig. 3).
Adding that he expected overall survival data to be available at the 2013 Genitourinary Cancers Symposium, Dr. Motzer noted that “overall survival is pretty much comparable at 1 year to the other agents”—77% for tivozanib vs 81% for sorafenib. Very few of the patients on tivozanib received subsequent second-line therapy, largely due to the patient’s geographic distribution.
According to immature data cited by Dr. Eisen, the percentage of patients receiving second-line treatment was 53% for sorafenib, “so it is really a sequential trial to a large extent—sorafenib, then tivozanib vs tivozanib alone, for the majority of patients, only 17% of whom went on to get any further treatment, which could have included agents other than tyrosine kinase inhibitors. The overall survival difference is worth noting. A plausible explanation would be that there is a difference in the second-line therapies, but plausible doesn’t necessarily mean correct. I think the onus is on us to provide further data to check that this is the case.”
‘Not Long to Wait’
“With regard to adverse events, tivozanib is a very safe drug, very tolerable in my own experience, which I think is well reflected from the TIVO-1 trial,” Dr. Motzer reported. All-grade adverse events occurred in 67.6% of patients receiving tivozanib vs 83.3% of those receiving sorafenib. Grade 3/4 adverse events were 36.3% vs 51.0%. For those receiving tivozanib, “the predominant side effects are hypertension and dysphonia,” Dr. Motzer said. “Other than that, it is a pretty well tolerated agent, and I think [physicians] will agree as [they] gain more experience with it.”
Tivozanib also demonstrated tolerability, “with a relatively low proportion of patients that require dose reductions or dose discontinuation on the study,” Dr. Motzer added. Dose reduction rates were 11.6% in the tivozanib group vs 42.8% in the sorafenib group, and dose discontinuation rates were 4.2% vs 5.4%.
Dr. Motzer “has made an excellent case for tivozanib,” Dr. Eisen said, noting that we shouldn’t use the phase II data to inform standard of care right now. Nevertheless, he added, “we don’t have long to wait.” ■
Disclosure: Dr. Rini has received research funding from and has been a consultant for Pfizer. Dr. Motzer has received research fundng from AVEO Oncology. Drs. Eisen and Porta reported no potential conflicts of interest.
References
1. Rini BI, Grünwald V, Fishman MN, et al: Axitinib for first-line metastatic renal cell carcinoma (mRCC): Overall efficacy and pharmacokinetic (PK) analyses from a randomized phase II study. 2012 ASCO Annual Meeting. Abstract 4503. Presented June 2012.
2. Escudier BJ, Porta C, Bono P, et al: Patient preference between pazopanib (Paz) and sunitinib: Results of a randomized double-blind, placebo-controlled, cross-over study in patients with metastatic renal cell carcinoma (mRCC)—PISCES study, NCT 01064310. 2012 ASCO Annual Meeting. Abstract CRA4502. Presented June 2, 2012.
3. Motzer RJ, Hutson TE, Reeves J, et al: Randomized open-label phase III trial of pazopanib versus sunitinib in first-line treatment of patients with metastatic renal cell carcinoma (MRCC): Results of the COMPARZ trial. 2012 ESMO Congress. Abstract LBA8. Presented October 1, 2012.
4. Goodman A: Pazopanib noninferior to sunitinib as front-line therapy for metastatic renal cell carcinoma. The ASCO Post. November 1, 2012.
5. Motzer RJ, Nosov D, Eisen T, et al: Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: Results from a phase III randomized, open-label, multicenter trial. 2012 ASCO Annual Meeting. Abstract 4501. Presented June 2012.