The need to identify a biomarker to guide treatment with bevacizumab (Avastin) is abundantly clear, and studies presented at the 2011 European Multidisciplinary Cancer Congress suggest this goal may be in sight.
“Finding predictive biomarkers is a challenge, but there are candidates,” said Eric Van Cutsem, MD, PhD, of the University Hospitals Leuven in Belgium.
 “Despite a global effort, there have been no tests to tell us which patients will benefit,” Gordon C. Jayson, MD, PhD, of the University of Manchester in the United Kingdom, agreed, but the availability of a more sensitive assay may change that, he said.
“Despite a global effort, there have been no tests to tell us which patients will benefit,” Gordon C. Jayson, MD, PhD, of the University of Manchester in the United Kingdom, agreed, but the availability of a more sensitive assay may change that, he said.
In close collaboration with Sanne de Haas, PhD, and Stefan Scherer, MD, PhD, from the Roche/Genentech Biomarker team, investigators analyzed blood samples from six randomized trials in various tumor types using a novel enzyme-linked immunosorbent assay (ELISA) that favors shorter vascular endothelial growth factor (VEGF)-A isoforms (VEGF121 and VEGF110), and they correlated plasma VEGF-A (pVEGF-A) with clinical outcomes. Median baseline levels of potential biomarkers were prespecified as a cutpoint to categorize patients as having low or high levels.
Pan-tumor Biomarker?
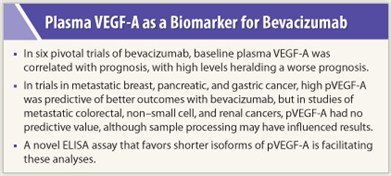 “Of several putative predictive biomarkers for bevacizumab, pVEGF-A is a lead candidate,” said Dr. Jayson.
“Of several putative predictive biomarkers for bevacizumab, pVEGF-A is a lead candidate,” said Dr. Jayson.
Plasma VEGF-A is potentially predictive (ie, correlated with outcome after bevacizumab therapy) and prognostic (correlated with outcome regardless of therapy) in metastatic breast, gastric, and pancreatic cancers, although it is prognostic only (not predictive) in metastatic colorectal cancer, non–small cell lung cancer, and renal cell carcinoma.
In his presentation, Dr. Jayson drew from analyses of the AVADO, AVAGAST, and AVITA trials, all demonstrating a predictive benefit for pVEGF-A in these tumor types.1 Overall survival in AVAGAST was greatest for patients with metastatic gastric cancer and high pVEGF-A receiving bevacizumab (HR = 0.72; P = .07). In AVADO, progression-free survival was greatest for metastatic breast cancer patients with high pVEGF-A receiving bevacizumab (HR = 0.49; P = .08); the marker was prognostic for overall survival in AVADO.
Predictive Value in Metastatic Pancreatic Cancer
 Dr. Van Cutsem, provided details from the AVITA trial in metastatic pancreatic cancer, in which pVEGF-A and also pVEGFR2 were both independently correlated with overall and progression-free survival.2 Other assessed angiogenic markers did not correlate with outcomes, he said.
Dr. Van Cutsem, provided details from the AVITA trial in metastatic pancreatic cancer, in which pVEGF-A and also pVEGFR2 were both independently correlated with overall and progression-free survival.2 Other assessed angiogenic markers did not correlate with outcomes, he said.
While high baseline pVEGF-A correlated with worse overall survival in the placebo arm, treatment with bevacizumab improved outcomes to put these patients on par with those with low baseline pVEGF-A (P = .03), Dr. Van Cutsem reported.
The hazard ratio for patients with high pVEGF-A levels was 0.56 while the hazard ratio was 1.02 for patients with low VEGF-A levels. A similar pattern was shown for progression-free survival with pVEGF-A, as well as with pVEGFR2 for progression-free and overall survival in pancreatic cancer.
Other Tumor Types
“Results with the novel assay showed the potential predictive value for pVEGF-A in breast, pancreatic, and gastric cancer. However, these findings were not replicated in metastatic colorectal cancer, non–small cell lung cancer, and renal cell carcinoma,” Dr. Jayson said.
 Data from the AVF2107g trial, AVAIL, and AVOREN, respectively, showed that pVEGF-A was prognostic but not predictive. Heavy crossover might have confounded the lack of correlation between the biomarker and overall survival in some tumor types (also in breast, in AVADO), he said.
Data from the AVF2107g trial, AVAIL, and AVOREN, respectively, showed that pVEGF-A was prognostic but not predictive. Heavy crossover might have confounded the lack of correlation between the biomarker and overall survival in some tumor types (also in breast, in AVADO), he said.
The value of the biomarker in NSCLC was also discussed at the meeting by Tony Mok, MD, of The Chinese University of Hong Kong. Dr. Mok presented a biomarker analysis of the ABIGAIL study, a global trial of first-line bevacizumab plus chemotherapy in advanced or recurrent nonsquamous non–small cell lung cancer.3 An exploratory analysis showed that there was a correlation between high baseline pVEGF-A levels and shorter progression-free survival (P = .002), but none of seven candidate biomarkers significantly correlated with overall response to the drug, he reported.
New Assay Aids in the Search
It is unclear why biomarker results seem different across tumor types. The difference in its predictive potential may be a reflection of variations in sample handling, Dr. Jayson suggested.
Citrate was used in colorectal cancer, non–small cell lung cancer, and renal cell carcinoma studies, while EDTA was used in breast, gastric, and pancreatic cancer studies. Samples in colorectal cancer and renal cell carcinoma were stored long-term, and samples from colorectal cancer, non–small cell lung cancer, and renal cell carcinoma were for a subset of samples subjected to at least two freeze-thaw cycles.
“Of course, we cannot exclude a true negative in colorectal cancer, non–small cell lung cancer, and renal cell carcinoma,” he added.
“Sample and assay characteristics may be critical in biomarker read-out,” he said, adding that correct storage duration and minimum freeze-thaw cycles could be important in quality control.
But the value of the novel assay in measuring pVEGF-A is evident, the speakers agreed. “The shorter VEGF-A isoform preference of the assay may play a critical role in these findings,” Dr. Van Cutsem said.
Problems with Analyses
According to Benjamin Besse, MD, PhD, of the Institut Gustave Roussy in Villejuif, France, circulating pVEGF-A is a prognostic factor, but the data are still insufficient to assess its predictive value. These were retrospective studies and small cohorts; in three of six trials, less than 50% of the serum was analyzed, he pointed out.
He added, “Bevacizumab was approved in 2004, and in 2011 there is still no predictive factor. These presentations are good news. But the findings need to be validated prospectively in clinical trials.”
As part of the bevacizumab biomarker program, prospective clinical trials are underway to explore pVEGF-A as a predictive marker, speakers responded. In the meantime, Dr. Jayson cautioned, “Don’t start measuring VEGF-A in your patients—yet!” ■
Disclosure: Dr. Van Cutsem has received research funding from Roche. Dr. Jayson has received research funding from Roche and has served on advisory boards for Roche.
References
1. Jayson GC, de Haas S, Delmar P, et al: Evaluation of plasma VEGFA as a potential predictive pan-tumour biomarker for bevacizumab. 2011 European Multidisciplinary Cancer Congress. Abstract 804. Presented September 24, 2011.
2. Van Cutsem E, Jayson G, Dive C, et al: Analysis of blood plasma factors in the AVITA phase III randomized study of bevacizumab with gemcitabine-erlotinib in patients with metastatic pancreatic cancer. 2011 European Multidisciplinary Cancer Congress. Abstract 803. Presented September 24, 2011.
3. Mok T, Gorbunova V, Juhasz E, et al: Biomarker analysis in BO21015, a phase II randomized study of first-line bevacizumab combined with carboplatin-gemcitabine or carboplatin-paclitaxel in paitents with advanced or recurrent non-squamous non-small cell lung cancer. 2011 European Multidisciplinary Cancer Congress. Abstract 9003. Presented September 24, 2011.

