The use of measurable (or minimal) residual disease (MRD) status to guide treatment in multiple myeloma has become a topic of intense interest. Phase III studies presented at the Plenary Session of the 2024 International Myeloma Society Annual Meeting moved MRD status ever closer to validation in the clinic.
The phase III IFM 2020-02 MIDAS trial is evaluating an MRD response–adapted strategy for consolidation therapy after induction, showing the feasibility of this approach along with a 95% response to the quadruplet regimen of isatuximab, carfilzomib, lenalidomide, and dexamethasone (Isa-KRd).1
The phase III AURIGA trial is looking at subcutaneous daratumumab plus lenalidomide as maintenance therapy after autologous stem cell transplant (ASCT) in patients who remain MRD-positive (ie, detectable MRD) after consolidation.2 Use of the two agents, as compared with lenalidomide alone, doubled the MRD-negative (ie, undetectable MRD) conversion rate and reduced the risk of disease progression or death by 47%.
Phase III IFM2020-02 MIDAS

Our findings confirm that six cycles of Isa-KRd induce exceptionally high response and MRD-negativity rates [in patients with multiple myeloma]…. These rates are the highest reported to date.— Aurore Perrot, MD, PhD
Tweet this quote
The Minimal Residual Disease Adapted Strategy (MIDAS) trial was designed to tailor therapy based on response and the achievement of MRD negativity after induction with six cycles of isa-KRd. The first efficacy and safety data were presented by Aurore Perrot, MD, PhD, -Professor of -Hematology at the Cancer University Institute Oncopole, -Toulouse, France.
“Our findings confirm that six cycles of Isa-KRd induce exceptionally high response and MRD-negativity rates, not only at a sensitivity of 10–5 but also at 10–6,” said Dr. Perrot. “These rates are the highest reported to date; regarding MRD negativity at 63%, it is favorably compared to CASSIOPEIA,3 GRIFFIN,4 and IsKia5 at 4 years.”
As she explained, in transplant-eligible patients with newly diagnosed multiple myeloma, induction with a quadruplet regimen before ASCT has become standard. To date, no prospective trials have compared upfront ASCT vs no transplant following quadruplet induction, leaving the role of upfront transplant a matter of debate. Risk-adapted strategies could help to determine its value, but none have been validated.

Sagar Lonial, MD
Sagar Lonial, MD, Professor and Chair of Hematology & Medical Oncology, Emory University School of Medicine, and Chief Medical Officer, Winship Cancer Institute, introduced MIDAS and noted that its novel trial design will set the standard for future trials. “The data presented here will help determine how we intermingle genetics and depth of response to shape a myeloma patient’s journey,” he said.
High Response Rates Postinduction
The study enrolled 791 patients from 72 centers, of whom 17% had high-risk cytogenetics. After induction, patients were separated into standard-risk and high-risk groups based on MRD negativity or positivity at 10–5 threshold; the standard-risk group is being randomly assigned to consolidation Isa-KRd with or without ASCT followed by lenalidomide maintenance, whereas the high-risk cohort is randomly assigned to ASCT plus Isa-KRd or tandem ASCT followed by isatuximab plus iberdomide as maintenance therapy. Stem cells were collected in 761 patients.
Among the 757 patients who completed induction with Isa-KRd, the overall response rate was 95%. In the intent-to-treat population, 92% of patients had a very good partial response or better and 64% to 66% of patients achieved near-complete or complete responses. These rates were even higher (99% and 69% to 71%, respectively) in the per-protocol population, Dr. Perrot reported.
Interesting Findings for Cytogenetic Risk
Postinduction MRD testing in the intent-to-treat population found MRD-negativity rates of 63% at 10–5 and 47% at 10–6; these rates were 66% and 50%, respectively, in the per-protocol population. Of note, MRD status after induction was not consistently aligned with initial cytogenetic risk (Table 1), Dr. Perrot said. Postconsolidation MRD results and kinetics are pending.
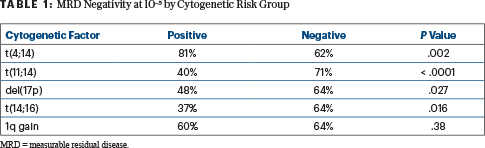
“Longer follow-up is needed to further understand the significance of achieving MRD negativity in particular subgroups,” she commented.
Phase III AURIGA

Ashraf Badros, MB, ChB
“To date, no randomized trial has directly compared daratumumab-based maintenance therapy vs standard lenalidomide maintenance in transplant-eligible newly diagnosed patients,” noted Ashraf Badros, MB, ChB, of the Greenbaum Cancer Center at the University of Maryland, Baltimore. Dr. Badros reported the primary results of AURIGA, which evaluated this approach in 200 transplant-eligible patients who were naive to anti-CD38 antibodies and remained MRD-positive after ASCT and consolidation. Patients were randomly assigned to subcutaneous daratumumab plus standard lenalidomide or lenalidomide alone. MRD was tested after 12, 18, 24, and 36 cycles. Median follow-up was 32 months, median treatment duration was 31 months for the combination and 21 months with lenalidomide alone, and all patients received at least 12 months of maintenance therapy.
The primary endpoint was MRD-negative conversion rate from baseline to 12 months, with the doublet demonstrating a strong benefit across the board (Table 2). “Daratumumab more than doubled the overall MRD-negative conversion rate and sustained MRD-negative rate,” he reported, adding that at a sensitivity of 10–6, the doublet quadrupled the conversion by 12 months and tripled it overall.
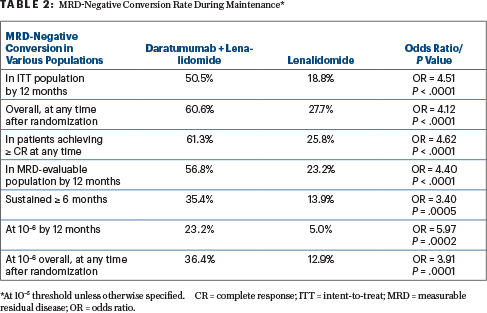
“MRD-negative conversion rates by 12 months were consistently higher with daratumumab plus lenalidomide vs lenalidomide alone across all clinically relevant subgroups…. It is important to note that not all patients have reached the 24-month point needed for MRD testing, and longer follow-up will determine whether higher MRD negativity rates will be observed,” he said.
Additional Positive Outcomes With Daratumumab/Lenalidomide Maintenance
The rate of complete response or better was higher with the doublet (75.8% vs 61.4%). Among patients with at least a very good partial response or better, the doublet deepened more responses to a complete or stringent complete response (66.2% vs 45.1%).
After 32.3 months, progression-free survival was 82.7% with the doublet and 66.4% with lenalidomide (hazard ratio [HR] = 0.53; P = .0361). Conversion to MRD negativity by 12 months was associated with improved progression-free survival, which at 30 months was 95.2% with the doublet and 94.1% with lenalidomide; in patients remaining MRD-positive, these rates were 69.0% and 59.3%, respectively, Dr. Badros reported.
EXPERT POINT OF VIEW

Luciano J. Costa, MD, PhD
Discussing the MIDAS and AURIGA trials for The ASCO Post was Luciano J. Costa, MD, PhD, the Mary and Bill Battle Professor in Multiple Myeloma and Director Multiple Myeloma Program at the University of Alabama at Birmingham. Dr. Costa led the MASTER trial,6 which used measurable residual disease (MRD) to inform the use and duration of daratumumab plus KRd (carfilzomib, lenalidomide, and dexamethasone) after autologous stem cell transplant (ASCT), allowing treatment to be discontinued in patients with two consecutive MRD-negative assessments. The MASTER-2 trial is now evaluating a response-adapted approach to consolidative therapy and the benefit of treating MRD-positive patients with a novel immunotherapy, the bispecific monoclonal antibody teclistamab, after combination induction.
Regarding the MIDAS results, Dr. Costa noted that 63% of patients achieved MRD negativity after induction therapy with isatuximab plus KRd and commented: “The importance of this large, 791-patient study is that it is the first randomized trial to deploy MRD response–adapted therapy, recognizing MRD postinduction as a great determinant of risk and asking different questions to MRD-defined subsets of patients. This is an important step toward personalized therapy, incorporating MRD in treatment decisions,” he said.
AURIGA asked whether adding daratumumab to lenalidomide for 2 years of maintenance would enhance the proportion of patients converting to MRD negativity vs proceeding with standard lenalidomide maintenance. Indeed, a higher proportion of MRD conversion was achieved with daratumumab plus lenalidomide (50.5% vs 18.8%), which translated into an improvement in progression-free survival.
“Although the findings may have limited application given that most patients currently receive an anti-CD38 monoclonal antibody as part of induction therapy, its importance cannot be overstated,” he commented. “AURIGA clearly demonstrates the feasibility of performing trials in an MRD-defined patient population and endorses MRD positivity as a starting point of therapy even in the absence of disease progression. Moreover, it elegantly demonstrates the principle that treatment augmentation informed by MRD status translates into improvement of clinically meaningful endpoints—in this case, progression-free survival.” n
DISCLOSURE: Dr. Perrot has served as a consultant for or received honoraria from AbbVie, Amgen, BMS, GSK, Janssen, Menarini-Stemline, Pfizer, Sanofi, and Takeda. Dr. Lonial has served as a consultant for or received research funding from Takeda, Novartis, Bristol Myers Squibb, GlaxoSmithKline, Amgen, Merck, Celgene, and Janssen. Dr. Badros reported no conflicts of interest. Dr. Costa has received honoraria from Amgen, Janssen, Pfizer, Adaptive Biotechnologies, and BMS; has served as a consultant or advisory board member for Amgen, BMS, Janssen, Pfizer, and Sanofi; and has received research support from Amgen, Janssen, BMS, Genentech, AbbVie, and Caribou.
REFERENCES
1. Perrot A, Touzeau C, Lambert J, et al: Efficacy and safety of Isa-KRd induction before response-adapted consolidation in transplant eligible newly diagnosed multiple myeloma: An interim analysis of the IFM2020-02 MIDAS study. 2024 International Myeloma Society Annual Meeting. Abstract OA-54. Presented September 27, 2024.
2. Badros A, Foster L, Anderson LD, et al: Subcutaneous daratumumab plus lenalidomide versus lenalidomide alone as maintenance therapy in newly diagnosed multiple myeloma after transplant: Primary results from the phase 3 AURIGA study. 2024 International Myeloma Society Annual Meeting. Abstract OA-45. Presented September 27, 2024.
3. Moreau P, Attal M, Hulin C, et al: Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 394:29-38, 2019.
4. Voorhees PM, Sborov DW, Laubach J, et al: Addition of daratumumab to lenalidomide, bortezomib, and dexamethasone for transplantation-eligible patients with newly diagnosed multiple myeloma (GRIFFIN): Final analysis of an open-label, randomised, phase 2 trial. Lancet Haematol 10:e825-e837, 2023.
5. Gay F, Roeloffzen W, Dimopoulos MA, et al: Results of the phase III randomized IsKia trial: Isatuximab-carfilzomib-lenalidomide-dexamethasone vs carfilzomib-lenalidomide-dexamethasone as pre-transplant induction and post-transplant consolidation in newly diagnosed multiple myeloma patients. Blood 142(suppl 1):4, 2023.
6. Costa LJ, Chhabra S, Medvedova E, et al: Daratumumab, carfilzomib, lenalidomide, and dexamethasone with minimal residual disease response-adapted therapy in newly diagnosed multiple myeloma. J Clin Oncol 40:2901-2912, 2022.

