Patients with untreated, metastatic BRAF-mutated melanoma may benefit from receiving immunotherapy first, moving to targeted therapy in the second line, data from the updated overall survival analysis of the randomized, phase II SECOMBIT trial suggest.1 The study aimed to define the optimal treatment sequencing in patients with BRAF mutations.

Paolo Ascierto, MD
“We can’t say definitively, because it’s a phase II noncomparative study, but the trend is very clear,” said lead author Paolo Ascierto, MD, who presented the findings at the European Society for Medical Oncology (ESMO) Congress 2021.
A “sandwich” strategy consisting of encorafenib/binimetinib for 8 weeks followed by ipilimumab/nivolumab for 8 weeks also proved more effective than starting with targeted therapy and reserving immunotherapy for the second line, he said.
Multiple studies have shown that in the first-line treatment of patients with BRAF V600E mutations, targeted therapy can yield high response rates, but they are often of limited duration. Immunotherapy (ipilimumab/nivolumab), conversely, is associated with lower but more durable response rates.
“The best sequencing has remained an open question,” said Dr. Ascierto, who is Director of the Unit of Melanoma, Cancer Immunotherapy, and Innovative Therapy for the National Tumour Institute “Fondazione G. Pascale” in Naples, Italy. “In addition, a short course of targeted therapy, then switching to ipilimumab/nivolumab at best response, is supported by models and may be clinically advantageous. To investigate the best sequential strategy, we started the SECOMBIT study, which is a randomized three-arm phase II study with no formal comparative test.”
SECOMBIT Details
The study enrolled 251 patients from 37 sites in 9 countries. All had metastatic melanoma harboring BRAF V600E mutations and were treatment-naive. Patients were randomly assigned to one of three treatment arms:
- Arm A: Targeted combination first with encorafenib at 450 mg plus binimetinib at 45 mg; upon disease progression, ipilimumab at 3 mg/kg plus nivolumab at 1 mg/kg;
- Arm B: Immunotherapy combination first with ipilimumab at 3 mg/kg plus nivolumab at 1 mg/kg; upon disease progression, encorafenib/binimetinib;
- Arm C: Sandwich approach starting with encorafenib/binimetinib for 8 weeks, then ipilimumab/nivolumab for 8 weeks; upon disease progression, more targeted treatment with encorafenib/binimetinib.
The arms were well balanced except for elevated lactate dehydrogenase [LDH], which was seen in 41% of Arm A, 48% of Arm B, and 36% of Arm C.
The study’s primary endpoint was overall survival. The current analysis was based on a database lock of May 31, 2021. Median follow-up was 32.2 months.
Survival Results
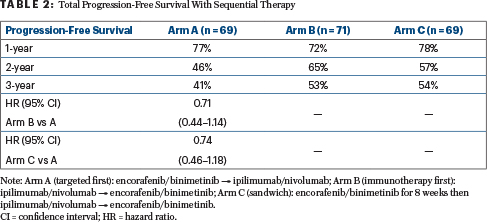
Dr. Ascierto presented the findings for overall survival (Table 1), the primary endpoint, and for total progression-free survival (Table 2), which was defined as the time from randomization until the date of second progression, noting that this is a “very good surrogate for overall survival.” Rates were reported for 1, 2, and 3 years.
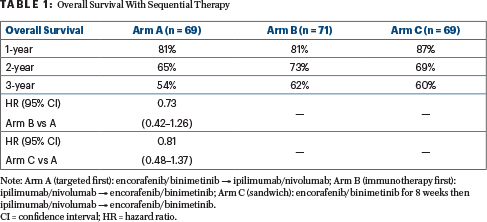
“Median overall survival was not reached in any arm, but it was interesting to see the survival rates at 2 years—65% with the targeted combination, 73% with combination immunotherapy, and 69% with the sandwich approach—and at 3 years. Interestingly, the odds ratios vs Arm A were 0.73 for ipilimumab/nivolumab first and 0.81 for the sandwich approach first. You see an interesting trend in favor of the combination immunotherapy given first,” Dr. Ascierto said.
Response Rates to First and Second Therapies
Dr. Ascierto noted that response rates to combination immunotherapy were 45% in the first line and 25% in the second line—so as second treatment, about half as many patients responded. With targeted therapy, the response rates in the second line were about 25% lower: 87% vs 61%.“If you look at the response rates to the first and second treatments, you see a lower rate for immunotherapy, by almost half, and this may be explained by resistance,” he said. He also noted that more patients treated first with targeted therapy discontinued treatment early due to toxicity; however, “many continued to respond.”
No new safety signals were identified with any of the treatment strategies, and profiles were comparable to what has been seen with ipilimumab/nivolumab and encorafenib/binimetinib. Grade 3 or 4 adverse events were most common in Arm B, with immunotherapy given first, but toxicities leading to treatment discontinuation were similar among the arms.
Application of the Findings
What do these findings mean for the emerging interest in triplet therapy in this disease setting (ie, a PD-1/L1 inhibitor plus BRAF inhibitor and MEK inhibitor)? The U.S. Food and Drug Administration approved atezolizumab plus vemurafenib and cobimetinib based on the IMspire150 trial, which demonstrated improved progression-free survival of 15.1 months with the triplet vs 10.6 months with vemurafenib/cobimetinib alone (P = .025).2
“Triplets are a hot topic” that may have a place in patients with symptomatic brain metastases, based on interesting data from the recent TRIDeNT study,3 Dr. Ascierto said. TRIDeNT evaluated nivolumab, dabrafenib, and trametinib and found median progression-free survival to be 8.5 months in patients with brain metastasis and 8.0 months in patients without them. Patients who experienced disease progression after adjuvant therapy may also be a reasonable target group for a triplet.
He also commented that the “sandwich” approach may be a good option for patients needing prompt impact on their disease burden—following up with immunotherapy for the long-term benefit.
Biomarker analyses are ongoing to learn more about the mechanisms of response and resistance to these three approaches.
DISCLOSURE: Dr. Ascierto has served as a consultant or advisor to Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, Array, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, Oncosec, Nouscom, Lunaphore, Seagen, and iTeos.
REFERENCES
1. Ascierto PA, Mandala M, Ferrucci PF, et al: SECOMBIT: The best sequential approach with combo immunotherapy (ipilimumab/nivolumab) and combo target therapy (encorafenib/binimetinib) in patients with BRAF mutated metastatic melanoma: A phase II randomized study. ESMO Congress 2021. Abstract LBA40. Presented September 20, 2021.
2. Gutzmer R, Stroyakovskiy D, Gogas H, et al: Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF V600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395:1835-1844, 2020.
3. Burton EM, Amaria RN, Glitza IC, et al: Phase II study of triplet combination nivolumab with dabrafenib and trametinib (TRIDeNT) in patients with PD-1 naive or refractory BRAF-mutated metastatic melanoma with or without active brain metastases. 2021 ASCO Annual Meeting. Abstract 9520. Presented June 4, 2021.

