As recently reported in The Lancet Oncology and reviewed in the October 10, 2020, issue of The ASCO Post, the phase III BROCADE3 trial has shown that the addition of veliparib to carboplatin and paclitaxel improved progression-free survival in previously treated BRCA-mutated advanced breast cancer.1 In the trial, veliparib was continued as maintenance therapy in patients who stopped carboplatin/paclitaxel for reasons other than disease progression.
It is not the first time that a more protracted cytotoxic therapy has been shown to extend progression-free survival in metastatic breast cancer. Back in 1998, the Eastern Cooperative Oncology Group focused on 195 patients in complete clinical remission after a first-line anthracycline-based regimen. These patients were randomly assigned to a multidrug regimen (cyclophosphamide, methotrexate, fluorouracil, prednisone, tamoxifen, and halotestin) or observation. The 10-month increase in the median time to relapse did not translate into a survival benefit.2

Martine Piccart, MD, PhD
Previous Trials of Maintenance Strategies
The IMELDA trial3 also tested a “switch” maintenance strategy in 185 patients with HER2-negative breast cancer and a response or disease stabilization after six cycles of docetaxel and bevacizumab. All patients continued bevacizumab, whereas half were given an oral, non–cross-resistant drug, namely capecitabine. Both the risk of disease progression and the risk of death were reduced by more than 50%. However, premature study closure, extensive censoring, lack of information on post-study treatment, and use of an agent no longer approved in many countries (bevacizumab) prevented this strategy from being adopted in routine clinical practice.
Maintenance chemotherapy with drugs that belong to the induction regimen has also extended progression-free survival. However, the results in terms of overall survival have been variable, and there has been no impact on treatment guidelines.
The Korean trial KCSG-BR07-02,4 which targeted a predominantly young patient population with any breast cancer subtype and in first metastatic relapse, randomly assigned 231 patients without disease progression after six cycles of 3-weekly paclitaxel plus gemcitabine to continuation of the same treatment vs observation; endocrine therapy was also given in case of hormone receptor–positive disease (41%). Median progression-free survival and overall survival were significantly improved by 3.7 months and 8.8 months, respectively, whereas quality of life was reported to be “similar.” Of note, a similar exposure to post-study treatment was documented in the two groups.
The GEICAM group5 was less successful in exploring maintenance liposomal doxorubicin vs observation after a response to a first-line regimen consisting of three cycles of doxorubicin followed by three cycles of docetaxel. Although a statistically significant 3.3-month improvement in time to disease progression was seen, overall survival was not impacted. Information on quality of life and post-study treatment is not available for the 155 randomly assigned patients.
In addition to often being underpowered for an overall survival endpoint, these trials suffer from “induction regimens” that moved quickly “out of fashion.”
Advantages to BROCADE3 Trial
The BROCADE3 trial has a number of distinct advantages in comparison with the previously mentioned studies. The trial targets a well-defined breast cancer population, namely women whose tumor is BRCA1- or BRCA2-mutated. It explores the addition of an oral, highly targeted drug—veliparib—to a popular induction regimen (intravenous carboplatin and paclitaxel) and as “maintenance therapy” in patients who stop induction chemotherapy for reasons other than disease progression.
The trial is double-blind and powered to detect a hazard ratio (HR) of 0.69 in progression-free survival favoring the veliparib-containing arm in a 2:1 randomized trial of 509 patients. It incorporates patient-outcome questionnaires based on “validated” tools, including the EORTC-QLQ-C30, the breast cancer–specific EORTC questionnaire, the Euro-QoL-5 Dimension 5-Level, and the Brief Pain Inventory.
Overall survival data are not yet “mature,” with 254 events observed out of 357 needed. BROCADE3 did reach its primary endpoint of a progression-free survival benefit (HR = 0.71, 95% confidence interval = 0.57–0.80) at the cost of a modest increase in grade 3 adverse events; it did not, however, demonstrate a clear benefit in quality of life.1 It is important to understand the particular features of the study population, namely the limited exposure to prior chemotherapy for advanced disease (19%) and to a platinum compound (8%). It is also important to notice the late divergence of the progression-free survival curves (after 10–12 months) as well as a “plateau” associated with a 15% higher probability of freedom of disease progression at 2 years for the veliparib-containing arm.
ESMO-MCBS: High Score for Veliparib
So, do we agree that BROCADE3 provides a new, compelling treatment option for women with advanced HER2-negative breast cancer and a germline BRCA mutation? To answer this question, I will rely on the European Society for Medical Oncology (ESMO)–Magnitude of Clinical Benefit Scale (MCBS), because it incorporates a structured, rational, and valid approach to data interpretation and analysis of randomized trials of new drugs/regimens in solid tumors that reduces the risk of “bias.”
“BROCADE3 receives an ESMO-MCBS score of 4, which ranks veliparib among the drugs with the highest scores in a palliative setting.”— Martine Piccart, MD, PhD
Tweet this quote
In an era of rapid expansion of new, expensive cancer medicines and in the context of cost-constrained health-care systems, ESMO embarked in 2013 on the development of a standard tool for grading the magnitude of clinical benefit of cancer drugs, which undergoes periodic revisions and is rapidly gaining in popularity.6,7 The aim is to emphasize those treatments that bring substantial benefits to patients and distinguish them from those with modest or even marginal benefits, providing a backbone for “value” evaluations by public policy makers, who will still need to add a cost-effectiveness dimension to the evaluation process. Applicable over a full range of solid tumors, the ESMO-MCBS highlights drugs that should ideally be prioritized for endorsement by health authorities across the European Union. Of note, ESMO-MCBS prioritizes direct endpoints such as overall survival and quality of life, which take precedence over less-reliable surrogates such as progression-free-survival or response rate.
It also gives credit to a plateau in the progression-free survival (or overall survival) curve, which is seen in BROCADE3. Based on all these considerations, BROCADE3 receives an ESMO-MCBS score of 4 (see Table 1), which ranks veliparib among the drugs with the highest scores in a palliative setting.
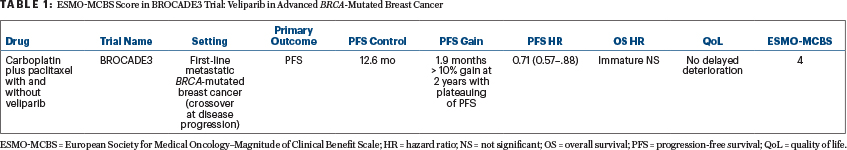
How could veliparib reach a score of 5 on the ESMO-MCBS? This can happen if improvements in quality of life are shown—which did not happen in the trial, unfortunately—or if an overall survival benefit of sufficient magnitude is seen, which will require longer follow-up. Although “crossover” to veliparib—currently documented in 44% of patients with progressive disease in the control arm—could attenuate an impact on overall survival, the provision for crossover is a strong feature of BROCADE3, as it makes the trial closer to a real-world setting.
What Next?
Should there be further investigations with veliparib in advanced BRCA-mutated breast cancer beyond BROCADE3? The answer is definitely yes! Challenging maintenance veliparib with maintenance platinum-based therapy would not be attractive, as it would impose more hospital visits for patients and likely negatively impact their quality of life. In contrast, exploring maintenance veliparib after a fixed number of carboplatin and paclitaxel cycles makes sense. I remain unconvinced that the progression-free survival observed in BROCADE3 is derived from both the combination of veliparib with chemotherapy and its continuation as maintenance therapy.
Investigators of BROCADE3 should be congratulated; they give us hope that the large randomized adjuvant OlympiA trial will generate positive results. This trial is exploring 1 year of olaparib after completion of (neo)adjuvant chemotherapy in “high-risk” women with BRCA- mutated cancers.
Dr. Piccart is employed at the Institut Jules Bordet, Brussels.
DISCLOSURE: Dr. Piccart has served as a consultant and/or received honoraria from AstraZeneca, Camel-IDS, Crescendo Biologics, Debiopharm, Genentech, Immunomedics, Lilly, Menarini, MSD, Novartis, Odonate, Pfizer, Radius, Roche, and Seattle Genetics; has received institutional research funding from AstraZeneca, Lilly, MSD, Novartis, Pfizer, Radius, Roche-Genentech, Servier, and Synthon; and has served as a board member for Oncolytics.
REFERENCES
1. Diéras V, Han HS, Kaufman B, et al: Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase III trial. Lancet Oncol 21:1269-1282, 2020.
2. Falkson G, Gelman RS, Pandya KJ, et al: Eastern Cooperative Oncology Group randomized trials of observation vs maintenance therapy for patients with metastatic breast cancer in complete remission following induction treatment. J Clin Oncol 16:1669-1676, 1998.
3. Gligorov J, Doval D, Bines J, et al: Maintenance capecitabine and bevacizumab vs bevacizumab alone after initial first-line bevacizumab and docetaxel for patients with HER2-negative metastatic breast cancer (IMELDA): A randomised, open-label, phase III trial. Lancet Oncol 15:1351-1360, 2014.
4. Park YH, Jung KH, Im SA, et al: Phase III, multicenter, randomized trial of maintenance chemotherapy vs observation in patients with metastatic breast cancer after achieving disease control with six cycles of gemcitabine plus paclitaxel as first-line chemotherapy: KCSG-BR07-02. J Clin Oncol 31:1732-1739, 2013.
5. Alba E, Ruiz-Borrego M, Margelí M, et al: Maintenance treatment with pegylated liposomal doxorubicin vs observation following induction chemotherapy for metastatic breast cancer: GEICAM 2001-01 study. Breast Cancer Res Treat 122:169-176, 2010.
6. Cherny NI, Sullivan R, Dafni U, et al: A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale. Ann Oncol 26:1547-1573, 2015.
7. Cherny NI, Dafni U, Bogaerts J, et al: ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol 28:2340-2366, 2017.

