In the phase III KEYNOTE-355 trial, pembrolizumab combined with several chemotherapy partners yielded a statistically significant and clinically meaningful improvement in progression-free survival vs chemotherapy alone in patients with previously untreated locally advanced or metastatic triple-negative breast cancer whose tumors have high expression of PD-L1. These findings were reported during the ASCO20 Virtual Scientific Program by Javier Cortes, MD, PhD, Head of Breast Cancer and Gynecological Cancers at Hospital Universitario Ramón y Cajal in Madrid and Clinical Investigator in the Breast Cancer Research Program at Vall d’Hebron Institute of Oncology in Barcelona.1

“The improvement in progression-free survival with pembrolizumab and chemotherapy was observed across patient subgroups, with a trend toward improved efficacy with PD-L1 enrichment.”— Javier Cortes, MD, PhD
Tweet this quote
“The improvement in progression-free survival with pembrolizumab and chemotherapy was observed across patient subgroups, with a trend toward improved efficacy with PD-L1 enrichment,” Dr. Cortes said. “Safety was consistent with the known profiles of each regimen, and no new safety signals were observed. These findings suggest a role for the addition of pembrolizumab to standard chemotherapy in the first-line treatment of metastatic triple-negative breast cancer.”
KEYNOTE Trials Paved the Way
Pembrolizumab monotherapy showed promising antitumor activity in patients with metastatic triple-negative breast cancer in the KEYNOTE-012, -086, and -119 trials, and responses were more durable than those with chemotherapy. In KEYNOTE-086, patients with positive PD-L1 expression seemed to derive more benefit than patients unselected for PD-L1 status; the PD-L1–positive subset had a 12-month overall survival rate of 61.7%.2
“The immunomodulatory properties of chemotherapy suggest that combining pembrolizumab with chemotherapy may enhance the antitumor activity,” said Dr. Cortes. Recently, in combination with standard neoadjuvant chemotherapy, pembrolizumab led to an almost 14% increase in pathologic complete responses in KEYNOTE-522.3 This led to a Breakthrough Therapy designation by the U.S. Food and Drug Administration for this strategy in the neoadjuvant setting for high-risk, early-stage, triple-negative disease.
KEYNOTE-355 Details
The phase III KEYNOTE-355 trial enrolled 847 patients (median age, 53 years) with previously untreated advanced or metastatic triple-negative breast cancer. The patients were randomly assigned 2:1 to pembrolizumab or placebo plus chemotherapy (nab-paclitaxel, paclitaxel, or gemcitabine/carboplatin) for up to 35 administrations. At baseline, 30% had de novo metastases, approximately 20% had a disease-free interval of less than 12 months, and 50% had a disease-free interval of at least 12 months.
Tumor PD-L1 status was a Combined Positive Score (CPS) ≥ 1 in 75% of patients and a CPS ≥ 10 in 36%. Patients were eligible for treatment, however, regardless of PD-L1 expression. Crossover was not allowed.
The dual primary endpoints were progression-free survival and overall survival by blinded central review in patients with a CPS ≥ 10 and a CPS ≥ 1 and in the overall (intention-to-treat) population.
The study had some statistical considerations: overall alpha controlled at one-sided (.025), split among progression-free survival (.005), overall survival (.018), and objective response rate (.002). Progression-free survival was tested using a hierarchical strategy, so pembrolizumab/chemotherapy had to prove to be superior to chemotherapy first in the group with a CPS ≥ 10 and then in patients with a CPS ≥ 1 to qualify for the intention-to-treat testing.
One Primary Endpoint Met
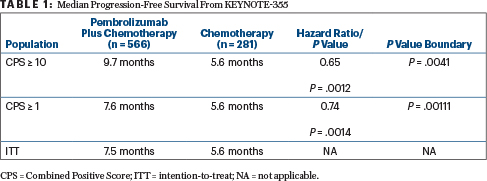
As of December 11, 2019, the median follow-up was 17.5 months for the pembrolizumab/chemotherapy arm (n = 566) and 15.5 months for chemotherapy alone (n = 281).
“Pembrolizumab plus chemotherapy significantly improved progression-free survival vs chemotherapy alone in patients with a CPS ≥ 10 tumors (Table 1), meeting one of the protocol-defined primary objectives. Although the boundary for a statistically significant benefit of pembrolizumab/chemotherapy in patients with a CPS ≥ 1 tumors was not met and formal testing in the intention-to-treat population was therefore not performed, the pembrolizumab treatment effect increased with PD-L1 enrichment,” Dr. Cortes said.
For the CPS ≥ 10 subset, 65% of the pembrolizumab arm was progression-free at 6 months, compared with 47% of the control arm. At 12 months, these rates were 39% and 23%, respectively, he reported.
“In patients with PD-L1 expression of a CPS ≥ 10, the benefit of pembrolizumab plus chemotherapy was generally consistent across most predefined subgroups…. Although its benefit seems not to be observed in patients with a disease-free interval less than 12 months, we should take into account the sample size is very small, the trial was not designed to look at this subgroup, and there were widely overlapping confidence intervals,” explained Dr. Cortes. “In patients with a CPS ≥ 1 as well, the benefit was generally consistent across subgroups..., and this was also maintained in the intention-to-treat population.”
KEY POINTS
- The phase III KEYNOTE-355 trial evaluated pembrolizumab plus chemotherapy vs chemotherapy alone in the first-line treatment of advanced or metastatic triple-negative breast cancer with any expression of PD-L1.
- In patients with tumor PD-L1 expression CPS ≥ 10, the combination significantly improved progression-free survival, from 5.6 months to 9.7 months (HR = 0.65;
P = .0041). - In a subgroup with a CPS ≥ 1 and in the intent-to-treat population, trends for improvement were shown.
Overall survival follow-up is ongoing. Grade 3 to 5 treatment-related adverse events were observed in 68.1% of the pembrolizumab arm (including two deaths, one of kidney injury and the other of pneumonia) and in 66.9% treated with chemotherapy alone. The rate of grade 3 or 4 immune-mediated toxicities and infusion reactions was 5.5% vs 0%, respectively. “Pembrolizumab plus chemotherapy was generally well tolerated, with no new safety concerns,” he said.
DISCLOSURE: Dr. Cortes holds stock or other ownership interests in MedSIR; has received honoraria from Celgene, Daiichi Sankyo, Eisai, Lilly, Merck Sharp & Dohme, Novartis, Pfizer, Roche, and Samsung; has served as a consultant or advisor to AstraZeneca, Athenex, Bioasis, Biothera, Boehringer Ingelheim, Celgene, Cellestia Biotech, Clovis Oncology, Daiichi Sankyo, Erytech Pharma, GlaxoSmithKline, Leuko, Lilly, Merck Sharp & Dohme, Merus, Polyphor, Roche, Seattle Genetics, and Servier; has received institutional research funding from Ariad, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer, Eisai Farmaceutica, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur, Puma CO, Queen Mary University of London, and Roche; and has been reimbursed for travel, accommodations, or other expenses by Daiichi Sankyo, Eisai, Novartis, Pfizer, and Roche.
REFERENCES
1. Cortes J, Cescon DW, Rugo HS, et al: KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy vs placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. ASCO20 Virtual Scientific Program. Abstract 1000.
2. Adams S, Loi S, Toppmeyer D, et al: Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: Cohort B of the phase II KEYNOTE-086 study. Ann Oncol 30:405-411, 2019.
3. Schmid P, Cortes J, Pusztai L, et al: Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020.

