In metastatic HER2-negative breast cancer, several trials have shown that the addition of the anti–vascular endothelial growth factor (VEGF) monoclonal antibody bevacizumab (Avastin) to different chemotherapy regimens significantly improved response rates and progression-free survival by various magnitudes compared to chemotherapy alone.1 Even though these trials failed to show an increase in overall survival with the addition of bevacizumab in metastatic disease, there has been considerable interest in evaluating bevacizumab in both the neoadjuvant and adjuvant settings.
Four Trials
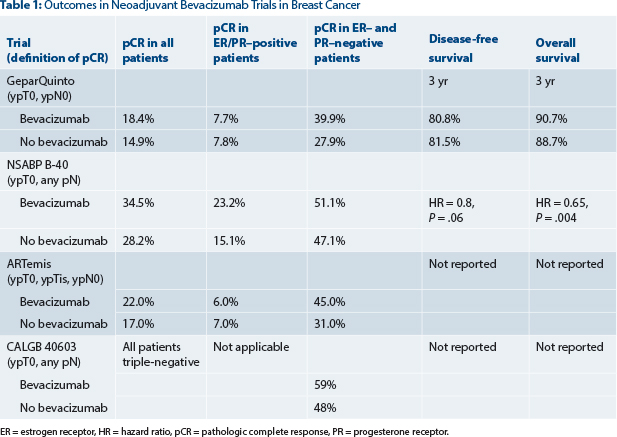
So far, we have data from four randomized phase III trials investigating bevacizumab in the neoadjuvant setting.2-5 All used pathologic complete response rate as the primary endpoint, albeit with different definitions of pathologic complete response (see Table 1).
The common observation in all four trials (GeparQuinto, National Surgical Adjuvant Breast and Bowel Project [NSABP] B-40, ARTemis, and Cancer and Leukemia Group B [CALGB] 40603) was that the pathologic complete response rate could be modestly but significantly increased with the addition of bevacizumab to anthracycline/taxane–based chemotherapy, with statistical significance being reached probably due to the high numbers of enrolled patients. Until now, survival outcome data were only available for the GeparQuinto trial, which showed no significant improvement in disease-free survival or overall survival with the addition of neoadjuvant bevacizumab among all patients or those with triple-negative disease.6
The role of bevacizumab in HER2-negative disease was also investigated in two big randomized phase III adjuvant trials7,8: The BEATRICE trial enrolled 2,591 patients with only triple-negative disease, and the E5103 study recruited 4,994 patients with HER2-negative disease and both hormone receptor–positive and hormone receptor–negative disease. In both trials, bevacizumab was added to chemotherapy, and after completing chemotherapy, patients were randomly assigned to receive or not receive single-agent bevacizumab for a total duration of 1 year. Neither trial showed improvement in disease-free survival or overall survival with the addition of 1 year of bevacizumab to adjuvant chemotherapy.
In contrast to the GeparQuinto study and the two big adjuvant trials, the recently published secondary outcome data of the neoadjuvant NSABP B-40 trial surprisingly reported a significant increase in overall survival (hazard ratio [HR] = 0.65, 95% confidence interval [CI] = 0.49–0.88, P = .004) with the addition of neoadjuvant plus adjuvant bevacizumab, with some evidence of greater benefit in hormone receptor–positive disease.9 The trial, reported by Bear and colleagues in the Lancet Oncology, is summarized in this issue of The ASCO Post.
Explaining the Different Results
Different trial designs and methodologic issues may explain the difference in the trial results. A major difference between the NSABP B-40 trial and the other neoadjuvant and adjuvant trials is the use of bevacizumab pre- and postoperatively. Whereas in the other trials, bevacizumab was given purely as either adjuvant or neoadjuvant treatment, patients in the NSABP B-40 trial received both neoadjuvant bevacizumab (for the first six cycles of chemotherapy) and adjuvant bevacizumab, for 10 additional doses every 3 weeks postoperatively.
Since bevacizumab is a targeted antiangiogenic agent, it is potentially less likely to eradicate micrometastatic disease—eg, in the bone marrow—whereas a manifest tumor in the breast, which is angiogenesis-dependent, may be more effectively treated by additional antiangiogenic therapy. This activity is reflected by the increased pathologic complete response rate with the use of bevacizumab in all neoadjuvant trials. Once the relatively short neoadjuvant treatment is completed, there might be a rebound of tumor cell growth.
Thus, continuous bevacizumab treatment after surgery might be a better strategy to prevent this phenomenon. However, the negative results of the adjuvant trials do not fully support this hypothesis. If the effect of bevacizumab in terms of overall survival in the NSABP B-40 is real, we may speculate that it is the combination of neoadjuvant and adjuvant application that is achieving this outcome.
Other Study Differences
Further differences among trials are that all trials used different chemotherapy backbones and that both the NSABP B-40 and CALGB 40603 trials had factorial designs in which modifications of the chemotherapy regimens were tested in parallel with the addition of bevacizumab. In the GeparQuinto trial, patients with no clinical response after the first four cycles of epirubicin and cyclophosphamide were removed from the study, potentially introducing bias. Moreover, the cutoff point for estrogen receptor–positive disease was different in the various trials. In addition, there are some major caveats in the interpretation of the NSABP B-40 trial. The endpoints of distant disease-free survival and overall survival were not prespecified, and thus there were no preplanned statistical calculations for these outcomes.
Further, disease-free survival was not significantly different in the B-40 trial between the bevacizumab and no-bevacizumab group (HR = 0.80, 95% CI = 0.63–1.01, P = .06). Since in almost all adjuvant trials, an improvement in overall survival is accompanied by an improvement in disease-free survival, this observation in the B-40 trial is difficult to explain.
Moreover, in the GeparQuinto and ARTemis trials, improved pathologic complete response rates were mainly observed in triple-negative patients but not in the hormone receptor–positive population. This finding is supported by the results of CALGB 40603 (which enrolled patients with triple-negative disease only). Contrary to these three studies, in the B-40 trial, the addition of bevacizumab increased the pathologic complete response rates in patients with hormone receptor–positive breast cancers but not in triple-negative disease. An exploratory analysis suggested somewhat greater benefit of bevacizumab among patients with hormone receptor–positive tumors, even though the interaction was not significant. No such difference regarding hormone-receptor status could be seen in the adjuvant E5103 trial, which included patients with both hormone receptor–positive and hormone receptor–negative disease.
Disappointing Findings
The so far contradictory or overall negative results of bevacizumab trials may come as a disappointment for many. Trials that investigate targeting drugs in populations without identified targets have been a large part of clinical research in the past 2 decades and have proven to be unsuccessful in most cases. Meta-analysis of such trials can identify the effects of bevacizumab in similar subgroups of the patient populations in the above-mentioned studies, but the heterogeneity of other patient characteristics, treatment regimens, and treatment durations in these trials will limit the validity and value of the findings.
Neoadjuvant trials offer a unique opportunity to investigate activity of different treatment regimens and, in conjunction, to harvest pre- and post-treatment tumor samples and to collect liquid biopsy samples. Translational studies allow us to look for predictors of efficacy (eg, with bevacizumab) and to gain further insight into the molecular mechanisms of action and resistance. Predictors of efficacy of bevacizumab identified in translational studies may foster new trials of this drug in defined subgroups. However, so far, there is still no role for bevacizumab in the adjuvant or neoadjuvant setting in patients with HER2-negative breast cancer outside of clinical trials. ■
Disclosure: Dr. Huober has received consultation fees from Roche and GlaxoSmithKline, research funding from GlaxoSmithKline, and travel support from Roche. Dr. Thürlimann holds stock in Roche and has received consultation honoraria and travel support from Roche.
References
1. Miles DW, Dieras V, Cortes J, et al: First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann Oncol 24:2773-2280, 2013.
2. von Minckwitz G, Eidtmann H, Rezai M, et al: Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med 366: 299-309, 2012.
3. Bear HD, Tang G, Rastogi P, et al: Bevacizumab added to neoadjuvant chemotherapy for breast cancer. N Engl J Med 366:310-320, 2012.
4. Earl HM, Hiller L, Dunn JA, et al: Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): An open-label, randomised, phase 3 trial. Lancet Oncol 16:656-666, 2015.
5. Sikov WM, Berry DA, Perou CM, et al: Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13-21, 2015.
6. von Minckwitz G, Loibl S, Untch M, et al: Survival after neoadjuvant chemotherapy with or without bevacizumab or everolimus for HER2-negative primary breast cancer (GBG 44-GeparQuinto). Ann Oncol 25:2363-2372, 2014.
7. Cameron D, Brown J, Dent R, et al: Adjuvant bevacizumab-containing therapy in triple-negative breast cancer (BEATRICE): Primary results of a randomised, phase 3 trial. Lancet Oncol 14:933-942, 2013.
8. Miller K, O`Neill AM, Dang CT, et al: Bevacizumab in the adjuvant treatment of HER2 negative breast cancer: Final results from Eastern Cooperative Oncology Group E5103. 2014 ASCO Annual Meeting. Abstract 500.
9. Bear HD, Tang G, Rastogi P, et al: Neoadjuvant plus adjuvant bevacizumab in early breast cancer (NSABP B-40 [NRG Oncology]): Secondary outcomes of a phase 3, randomised controlled trial. Lancet Oncol 16:1037-1048, 2015.
Dr. Huober is Professor and Head of the Breast Center, Department of Gynecology and Obstetrics, University of Ulm, Germany, and Dr. Thürlimann is Professor and Head of the Breast Center, Cantonal
Hospital St. Gallen, Switzerland.