For advanced/metastatic melanoma patients with BRAF mutations, two pathway inhibitors are better than one, according to studies presented at the European Society for Medical Oncology (ESMO) 2014 Congress that demonstrated improved progression-free and overall survival for regimens combining a BRAF inhibitor with an inhibitor of the MEK protein.
“The two combination trials presented here today are compelling. We have at least two combination therapies that look beneficial,” commented Jeffrey S. Weber, MD, PhD, Senior Member of the Moffitt Cancer Center and Director of the Donald A. Adam Comprehensive Melanoma Center in Tampa, Florida, at an ESMO press briefing. Dr. Weber presented a separate study of nivolumab vs chemotherapy in previously treated patients, showing that 95% of responders to the immunotherapy were still in remission at 24 weeks.1
The combination of a BRAF inhibitor and a MEK inhibitor appears to mitigate the emergence of disease resistance that occurs with BRAF inhibition alone and ameliorate cutaneous toxicity. The COMBI-v and coBRIM studies, both in previously untreated BRAF mutation–positive patients and both presented at the ESMO Presidential Symposium, supported these concepts.
“Studies show the combination should be used instead of a BRAF inhibitor as a single agent. The data are very convincing,” concluded Caroline Robert, MD, PhD, Head of the Dermatology Unit at the Institut Gustave-Roussy, Villejuif, France, at a press briefing.
Dabrafenib Plus Trametinib
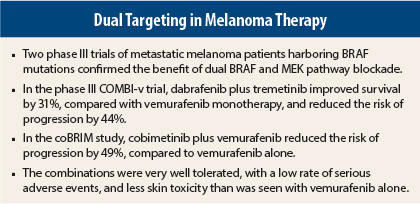
The COMBI-v trial compared the combination of the BRAF inhibitor dabrafenib (Tafinlar) at 150 mg twice daily plus the MEK inhibitor trametinib (Mekinist) at 2 mg/d with vemurafenib (Zelboraf) monotherapy (960 mg twice daily) in 704 treatment-naive patients with the BRAF V600 mutation.2 Overall survival was the primary endpoint of the study.
“The Independent Data Monitoring Committee recommended stopping the study based on an interim analysis that demonstrated an overall survival benefit that crossed the prespecified efficacy stopping boundary for the combination,” announced Dr. Robert at the press briefing. “We saw a significant improvement in all endpoints.”
The preplanned interim analysis found a 31% reduction in mortality among patients on the combination, and a 44% reduction in the risk of progression or death, compared to vemurafenib monotherapy. After a median follow-up of 11 months, median overall survival was 17.2 months with vemurafenib alone and was not reached in the dabrafenib/trametinib arm (HR = 0.69; P = .005).
“The curves separated early, at 3 months. The P value was clearly below the prespecified boundary (P < .0214),” Dr. Robert noted.
Overall response rates were 64% for the combination (with 13% complete responses) vs 51% for vemurafenib (with 8% complete responses).
Median progression-free survival was 11.4 months for dabrafenib/trametinib recipients and 7.3 months for the vemurafenib group (hazard ratio [HR] = 0.56; P < .001); response rates were 64% and 51% (P < .001), and duration of response was 13.8 vs 7.5 months, respectively.
Patients tolerated the combination well, and the rates of adverse events and serious adverse events were generally similar in the two arms. “In fact, grade 3 toxicities were actually lower with the combination (48%) than with single-agent vemurafenib (57%),” Dr. Robert noted.
Cutaneous malignancies occurred in 1% of the experimental arm vs 18% of the vemurafenib arm, and hyperproliferative events and photosensitivity were also less frequent with the two drugs.
Vemurafenib Plus Cobimetinib
Vemurafenib (960 mg twice daily) plus the MEK inhibitor cobimetinib (60 mg/d) was compared to vemurafenib alone, in 28-day cycles, in the coBRIM study of 495 treatment-naive patients.3 The data were presented at the meeting by Grant McArthur, MD, Head of the Cancer Therapeutics Program at the Peter MacCallum Cancer Centre in Melbourne.
The study’s primary endpoint was met when the combination arm achieved a median progression-free survival of 9.9 months, compared to 6.2 months with vemurafenib alone (HR = 0.51, P < .0001)—a 49% reduction in the risk of progression, according to investigator-assessed review. The independent review analysis was similar (HR = 0.60, P = .0003). Response rates were 68% vs 45% (P < .0001), with complete responses achieved by 10% vs 4%, respectively—differences deemed “striking” by Dr. McArthur.
The interim analysis of overall survival found a 35% reduction in deaths (HR = 0.65; P < .046). Median overall survival was not reached in either arm, but 9-month survival rates were 81.1% and 72.5%, Dr. McArthur reported.
The toxicity profile was consistent with previous trials. Gastrointestinal side effects were more common with the combination, but were primarily grade 1. Photosensitivity reaction was also more common, but cutaneous squamous cell carcinoma and keratoacanthomas were significantly less frequent with the two drugs. Serous retinopathy occurred in 51 patients receiving the combination (20%) vs one patient on vemurafenib monotherapy.
Toxicities of at least grade 3 were observed in 65% of the combination arm and 59% of the monotherapy arm, and discontinuations due to treatment were similar (approximately 12%).
“This study provides clear and definitive evidence that cobimetinib combined with vemurafenib results in improved progression-free survival and objective response rates, and our preliminary overall survival analysis is promising,” Dr. McArthur noted at a press briefing. “We are adding a second agent to a very active drug, vemurafenib, and we still get striking results.”
“We anticipate that the combination of a BRAF and MEK inhibitor will become a new standard treatment for advanced BRAF-mutant melanoma,” he said. “The data lay the foundation for the addition of treatments, either in sequence or in further combination, to obtain even better results.” ■
Disclosure: Dr. Weber disclosed receiving honoraria from Bristol-Myers Squibb, Merck, Genentech, AstraZeneca, and AbbVie; clinical research funding from Bristol-Myers Squibb, Merck, GlaxoSmithKline, and Macrogenics (to Moffitt Cancer Center); serving as an advisor to Ichor Therapeutics, Lion Biotechnologies, and Pieris, and owning stock in Celldex Therapeutics, Altor BioScience, and cCAM Biotherapeutics. Dr. Robert has received fees for advisory board participation from Bristol-Meyers Squibb, Roche, Merck, GlaxoSmithKline, Novartis, and Amgen. Dr. McArthur has received research support from Roche, Novartis, Pfizer, Millennium, and Celgene and is a consultant for Provectus.
References
1. Weber JS, Minor DR, D’Angelo SP, et al: A phase 3 randomized, open-label study of nivolumab versus investigator’s choice chemotherapy in previously treated advanced melanoma. ESMO 2014 Congress. Abstract LBA3_PR. Presented September 29, 2014.
2. Robert C, Karaszewska B, Schachter J, et al: COMBI-v: A randomized, open-label, phase III study comparing the combination of dabrafenib and trametinib with vemurafenib as first-line therapy in patients with unresectable or metastatic BRAF V600E/K mutation-positive cutaneous melanoma. ESMO 2014 Congress. Abstract LBA4_PR. Presented September 29, 2014.
3. McArthur GA, Ascierto PA, Larkin J, et al: Phase 3, double-blind, placebo-controlled study of vemurafenib versus vemurafenib + cobimetinib in previously untreated BRAF V600 mutation-positive patients with unresectable locally advanced or metastatic melanoma. ESMO 2014 Congress. Abstract LBA5_PR. Presented September 29, 2014.