In the phase I/II KRYSTAL-1 trial, the KRAS inhibitor adagrasib demonstrated clinical activity in previously treated patients with pancreatic ductal adenocarcinoma, biliary tract cancer, and other solid tumors harboring KRAS G12C mutations, according to research presented at the ASCO Plenary Series April 2023 session.1 The findings came from an updated analysis of one arm of the multicohort study, after a median follow-up of 16.8 months.
“Adagrasib monotherapy demonstrated clinically meaningful activity in a variety of KRAS G12C–mutated solid tumors, for which no standard-of-care treatment options are available,” said Shubham Pant, MD, Professor in the Department of Gastrointestinal Medical Oncology; Director of Clinical Research; and Associate Director for Early-Phase Drug Development at The University of Texas MD Anderson Cancer Center in Houston.
The KRYSTAL-1 cohort is the largest phase II tumor-agnostic data set to evaluate KRAS G12C–mutated solid tumors, excluding non–small cell lung cancer (NSCLC) and colorectal cancer, he indicated.

Shubham Pant, MD
Long-Standing Need to Target KRAS G12C
KRAS, a mediator of signaling pathways essential for cellular growth, proliferation, and survival, is the most frequently mutated oncogene in cancer. The KRAS G12C mutation acts as an oncogenic driver in a range of solid tumors, particularly NSCLC (14%). Other sites are colorectum (3%–4%), appendix (3%–4%), small bowel (1%–3%), biliary tract (1%), pancreas (1%–3%), endometrium (1.5%), and ovaries (0.4%).
Adagrasib is a covalent KRAS G12C inhibitor that irreversibly and selectively binds KRAS G12C in its inactive state. It has several favorable properties, including a long half-life (23 hours), dose-dependent pharmacokinetics, and central nervous system penetration. The drug was granted accelerated approval by the U.S. Food and Drug Administration for the treatment of KRAS G12C–mutated NSCLC and received Breakthrough Therapy designation, given in combination with cetuximab, for KRAS G12C–mutated colorectal cancer.
KRYSTAL-1 Details
KRYSTAL-1 is an ongoing multicohort phase I/II trial evaluating adagrasib in patients with advanced solid tumors harboring KRAS G12C mutations. There are multiple cohorts in the full population with a variety of tumors and treated with different doses (sometimes in combination with other agents).
Dr. Pant’s presentation focused on a phase II cohort with unresectable or metastatic KRAS G12C–mutated solid tumors (excluding NSCLC and colorectal cancer) receiving adagrasib monotherapy orally at 600 mg twice daily.
As of October 1, 2022, there were 64 patients enrolled into this arm, including 21 patients with pancreatic cancer, 12 with biliary tract cancer, 10 with appendiceal cancer, 5 with ovarian cancer, 4 with cancers of the esophagus or gastroesophageal junction, 4 with unknown primaries, 3 with small bowel cancer, 3 with endometrial cancer, and 1 each with breast cancer and glioblastoma. Their median prior lines of systemic therapy were two, but 34% had received at least three, and 8% had no prior therapy.
Activity Observed
Efficacy outcomes were available for 57 patients with measurable disease who received at least one dose and were followed for a median 16.8 months. The objective response rate by blinded independent central review was 35.1%, all partial responses. The disease control rate was 86.0%, and the median duration of response was 5.3 months. Median progression-free survival was 7.4 months, and median overall survival was 14.0 months. Activity varied according to tumor type (Table 1).
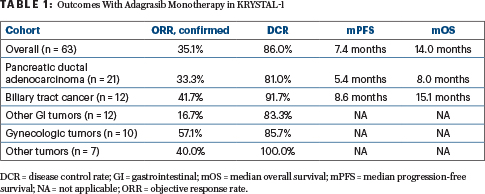
Adagrasib was particularly active in biliary tract cancer (Table 1). At 1 year, 14.6% of this cohort were progression-free, and 87.5% were alive. The subset with pancreatic cancer was also favored.
“The clinical activity of adagrasib in patients with pancreatic and biliary tract cancers is noteworthy, as chemotherapy has limited clinical activity in these patient populations in the second-line setting,” he remarked.
Safety Profile
Treatment-related adverse events of any grade were observed in 96.8% of patients, but they were primarily grades 1 and 2, mostly nausea (49.2%), diarrhea (47.6%), fatigue (41.3%), and vomiting (39.7%). Grade 3 treatment-related adverse events occurred in 25.4% of patients; one (1.6%) experienced grade 4 febrile neutropenia.
Treatment-related adverse events led to dose reductions in 25 patients (39.7%) and dose interruptions in 28 patients (44.4%). None led to treatment discontinuation, and no patient died because of treatment, Dr. Pant said.
DISCLOSURE: Dr. Pant has served as a consultant or advisor to Ipsen, Janssen, Boehringer Ingelheim, Ask Gene Pharma, Novartis, and Zymeworks and has received institutional research funding from various companies.
REFERENCE
1. Pant S, Yaeger R, Spira AI, et al: KRYSTAL-1: Activity and safety of adagrasib (MRTX849) in patients with advanced solid tumors harboring a KRAS G12C mutation. ASCO Plenary Series. Abstract 425082. Presented April 20, 2023.

