After resolving missing survival data in the phase III ENGOT-OV16/NOVA trial, no statistically significant difference in overall survival was found for patients with platinum-sensitive recurrent ovarian cancer who received maintenance therapy with the PARP inhibitor niraparib, investigators reported at the Society of Gynecologic Oncology (SGO) 2023 Annual Meeting on Women’s Cancer.1
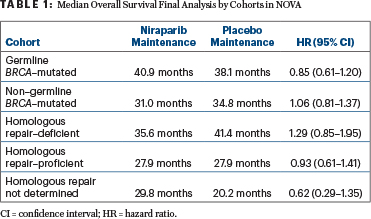
Once investigators obtained survival data that had been lacking for 92 patients, the survival differences between the niraparib and placebo arms “were not interpreted to be significant” in either the cohort with germline BRCA mutations or the non–germline BRCA–mutated cohort, nor in any of the exploratory homologous recombination subgroups, though the hazard ratio (HR) numerically favored niraparib in the germline BRCA–mutated population (Table 1), according to Ursula A. Matulonis, MD, Chief of the Division of Gynecologic Oncology, Dana-Farber Cancer Institute, and Professor of Medicine, Harvard Medical School.
These data were previously released by GlaxoSmithKline on November 11, 2022, after which the U.S. Food and Drug Administration (FDA) recommended that niraparib maintenance for the second-line or later setting be restricted to germline BRCA–mutated patients. A similar restriction occurred on December 12, 2022, for rucaparib maintenance to only tumor BRCA-mutated ovarian cancer because of survival trends seen in ARIEL3,2 “with hazard ratios not going in the right direction,” Dr. Matulonis told The ASCO Post. “The European Medicine Agencies, on the other hand, have kept the full indications.”
In the NOVA cohort with germline BRCA–mutated tumors, median overall survival was 40.9 months in the niraparib arm and 38.1 months in the placebo arm (HR = 0.85; 95% confidence interval [CI] = 0.61–1.20). In the non–germline BRCA–mutated cohort, median overall survival was 31.0 months and 34.8 months, respectively (HR = 1.06; 95% CI = 0.81–1.37). The wide confidence intervals were as expected, she noted, given that NOVA was not powered for a formal overall survival analysis, which was a secondary endpoint.
Dr. Matulonis emphasized the challenge in interpreting the findings of this final overall survival analysis. “NOVA’s long-term follow-up data analyses were confounded by imbalances and postprogression therapy, including subsequent PARP inhibitor use by treatment arms in both the germline BRCA–mutated and non–germline BRCA–mutated cohorts, including all subgroups of homologous recombination,” she noted.
The results of NOVA, she added, underscore the importance of long-term follow-up of patients and point to a continued need for research.
“Our patients with ovarian cancer are living longer and are thus receiving more therapies,” said Dr. Matulonis. “It is critical for trials to follow patients long term for overall survival after they stop study treatment and to carefully record and chronicle poststudy treatment therapies. Understanding the impact of PARP inhibitor therapy on postprogression treatment resistance is also an important area of research.”
About the NOVA Trial
ENGOT-OV16/NOVA was a randomized, double-blind, placebo-controlled, phase III trial that enrolled 553 patients with platinum-sensitive recurrent ovarian cancer. Patients were enrolled into independent germline BRCA–mutated and non–germline BRCA–mutated cohorts and then randomly assigned 2:1 to receive niraparib at 300 mg once daily or placebo after standard platinum therapy. Patients were stratified by progression-free survival (6–12 months vs ≥ 12 months), best response to last platinum-based therapy, and prior bevacizumab. The primary endpoint was progression-free survival by blinded independent central review.
Primary results from the study, released in 2016,3 indicated a statistically significant progression-free survival benefit for niraparib maintenance compared with placebo maintenance in the key subsets, including germline BRCA–mutated (HR = 0.27), non–germline BRCA–mutated (HR = 0.45), homologous repair–deficient (HR = 0.38), and homologous repair–proficient (HR = 0.58) populations. Long-term analyses of the second progression-free survival (PFS2) also indicated the benefit of maintenance niraparib beyond the first disease progression, but overall survival analyses, a secondary endpoint, were limited by missing data.4
Missing Data Retrieved for New Analysis

Ursula A. Matulonis, MD
“The preplanned overall survival analysis for NOVA was presented previously at SGO 2021 but was associated with missing data on survival status and postprogression therapies. After the mature overall survival analysis was presented to the FDA, the agency recommended retrieval of further data. Data retrieval efforts reduced the missing survival status from 17% down to about 2%, and the data cutoff was extended by 6 months to the date of study unblinding. Final overall survival was evaluated in both cohorts and by homologous repair status in the non–germline BRCA–mutated cohort as an exploratory analysis.
For the updated analysis, in which overall survival is now 77.9% mature, vital status procedure was completed to retrieve the last known alive status for 92 patients with missing survival data, a process that included outreach to 24 sites across 13 countries. As of the data cutoff of March 31, 2021, this resulted in known survival status for 97.6% of patients in this updated analysis," Dr. Matulonis said.
Other Outcome Data
Although the overall survival data were not significant, Dr. Matulonis noted that additional secondary endpoints—including chemotherapy-free interval, time to first subsequent therapy, PFS2, and time to second subsequent therapy—demonstrated “a persistent treatment effect numerically in favor of niraparib vs placebo in both the germline BRCA–mutated and non–germline BRCA–mutated cohorts.”
Of note, in the germline BRCA–mutated cohort, median chemotherapy-free interval was 20.0 months with niraparib vs 9.4 months with placebo (HR = 0.39), median time to first subsequent therapy was 19.1 months vs 8.6 months (HR = 0.57), PFS2 was 29.9 months vs 22.7 months (HR = 0.70), and time to second subsequent therapy was 29.7 months vs 19.6 months (HR = 0.63).
The safety profile in NOVA was consistent with that observed in previous data readouts. No new safety signals were observed. As of the March 31, 2021, data cutoff, 3.8% of patients receiving niraparib and 1.7% receiving placebo developed myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). The risk for these malignancies was highest in the germline BRCA–mutant cohort treated with a PARP inhibitor and was 7.4%, consistent with other phase III studies in the recurrent platinum-sensitive setting.
DISCLOSURE: Dr. Matulonis has served as a consultant or advisor for Agenus, AstraZeneca, Blueprint Medicines, Immunogen, Allarity, Boehringer Ingelheim, CureLab, GlaxoSmithKline, Merck, NextCure, Novartis, and Trillium; and is serving on data and safety monitoring boards for Alkermes and Symphogen.
REFERENCES
1. Matulonis UA, Herrstedt J, Oza A, et al: Final overall survival and long-term safety in the ENGOT-OV16/NOVA phase III trial of niraparib in patients with recurrent ovarian cancer. Society of Gynecologic Oncology 2023 Annual Meeting on Women’s Cancer. Presented March 25, 2023.
2. Coleman RL, Oza AM, Lorusso D, et al: Overall survival results from ARIEL3: A phase 3 randomized, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma. 2022 International Gynecologic Cancer Society. Abstract O003. Presented September 29, 2022.
3. Mirza M, Monk BJ, Herrstedt J, et al: Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer. N Engl J Med 375:2154-2164, 2016.
4. Matulonis U, Herrstedt J, Oza A, et al: Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecol Oncol 162(suppl 1):S24-S25, 2021.

