A rapid update to the ASCO Guideline on neoadjuvant therapy for breast cancer adds a recommendation on the use of pembrolizumab in patients with high-risk early-stage triple-negative breast cancer.1 The update follows a recent analysis from the randomized phase III KEYNOTE-522 trial that showed a significant event-free survival benefit with the addition of pembrolizumab to standard neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery in patients with high-risk triple-negative breast cancer.2
The results from this study led to the U.S. Food and Drug Administration (FDA) approval of pembrolizumab in this setting.3 “This is the first FDA approval of an immunotherapy agent in early-stage breast cancer,” noted Larissa A. Korde, MD, MPH, FASCO, of the National Cancer Institute, and Guideline Co-Chair.

Larissa A. Korde, MD, MPH, FASCO
Updated Recommendation
In the 2022 ASCO Guideline update, pembrolizumab at 200 mg every 3 weeks or 400 mg every 6 weeks is recommended in combination with neoadjuvant chemotherapy, followed by adjuvant pembrolizumab after surgery, for patients with T1cN1–2 to T2–4N0 (stage II/III) early-stage triple-negative breast cancer.1 Adjuvant pembrolizumab may be given concurrently with, or after completion of, radiation therapy. Under the previous version of the guideline, developed before these data were available, there was insufficient evidence to support adding immune checkpoint inhibitors to standard neoadjuvant chemotherapy for patients with triple-negative breast cancer.4 Thus, this rapid update provides an important revision to that recommendation.
Evidence for Guideline Update
KEYNOTE-522 was a randomized, multicenter, double-blind, placebo-controlled trial designed to compare neoadjuvant carboplatin and paclitaxel followed by doxorubicin or epirubicin and cyclophosphamide plus either pembrolizumab (784 patients) or placebo (390 patients), followed by pembrolizumab or placebo administered every 3 weeks for up to nine cycles after surgery, in patients with previously untreated stage II/III triple-negative breast cancer.2
After a median follow-up of 39.1 months, pembrolizumab plus neoadjuvant chemotherapy followed by adjuvant pembrolizumab after surgery was associated with a significant improvement in event-free survival over placebo plus chemotherapy, with 3-year event-free survival rates of 84.5% and 76.8%, respectively (hazard ratio = 0.63, 95% confidence interval = 0.48–0.82; P < .001). The overall survival data are not yet mature; 3-year overall survival rates are 89.7% and 86.9%, respectively.
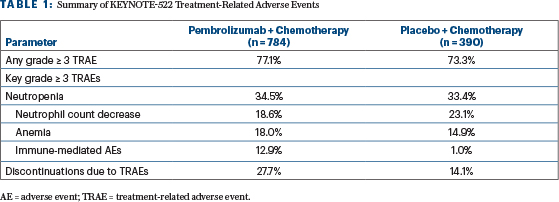
The most frequent grade ≥ 3 treatment-related adverse events were hematologic (Table 1). Endocrine disorders, including hypo- or hyperthyroidism, adrenal insufficiency, thyroiditis, and hypophysitis, occurred more frequently in the pembrolizumab/chemotherapy arm than in the placebo/chemotherapy arm, at 26.8% and 9.1%, respectively.
“Careful attention should be paid to screening and following patients for these side effects that can be lifelong among patients being treated with pembrolizumab,” said Dawn L. Hershman, MD, MS, FASCO, of the Herbert Irving Comprehensive Cancer Center at Columbia University Medical Center, and Guideline Co-Chair. The guideline specifies the need for careful screening for, and management of, common toxicities, given that immune-related adverse events from pembrolizumab can be severe and permanent.

Dawn L. Hershman, MD, MS, FASCO
Ongoing Questions
Looking ahead, an area of interest is whether the event-free survival benefit with neoadjuvant and adjuvant pembrolizumab translates into an improvement in overall survival with continued follow-up. Dr. Korde noted that if the final KEYNOTE-522 overall survival analysis does show a significant survival benefit with pembrolizumab that “this would be an important step forward for patients with triple-negative breast cancer.” The guideline authors also note that uncertainty remains concerning the optimal adjuvant treatment for these patients. Clinical trials have demonstrated an independent benefit with adjuvant capecitabine in patients with triple-negative breast cancer and with adjuvant olaparib in patients with BRCA germline mutations, each without pembrolizumab.5,6 There are no data to support the use of pembrolizumab in combination with either capecitabine or olaparib.
REFERENCES
1. Korde LA, Somerfield MR, Hershman DL, et al: Use of immune checkpoint inhibitor pembrolizumab in the treatment of high-risk, early-stage triple negative breast cancer: ASCO guideline rapid recommendation update. J Clin Oncol. April 13, 2022 (early release online).
2. Schmid P, Cortes J, Dent R, et al: Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med 386:556-567, 2022.
3. U.S. Food and Drug Administration: FDA approves pembrolizumab for high-risk early-stage triple-negative breast cancer. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-high-risk-early-stage-triple-negative-breast-cancer. Accessed March 22, 2022.
4. Korde LA, Somerfield MR, Carey LA, et al: Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39:1485-1505, 2021.
5. Masuda N, Lee SJ, Ohtani S, et al: Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 376:2147-2159, 2017.
6. Tutt ANJ, Garber JE, Kaufman B, et al: Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 384:2394-2405, 2021.
Originally published in ASCO Daily News. © American Society of Clinical Oncology. ASCO Daily News, April 20, 2022. All rights reserved.

