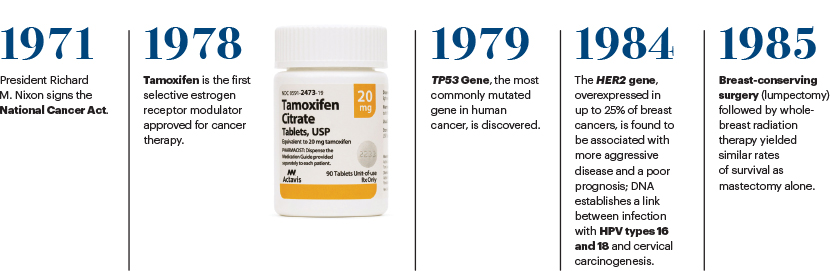
By the time President Richard M. Nixon signed the National Cancer Act into law on December 23, 1971, cancer had become the nation’s second leading cause of death; only about one of two people diagnosed with cancer survived at least 5 years—compared to two of three people diagnosed with the disease today.1 Throughout the decade, survivorship numbers remained dismally low, hovering around 3 million.2 Driven by public fear that cancer was contagious, the disease was so stigmatized, the word “cancer” was rarely uttered out loud, and it was common practice for physicians to avoid revealing and discussing a cancer diagnosis with patients.3

When President Nixon signed the National Cancer Act, which has come to be called his “war on cancer,” into law, he said: “I hope in the years ahead we will look back on this action today as the most significant action taken during my administration.”4 The unprecedented legislation granted sweeping authority to the director of the National Cancer Institute (NCI) to develop a national cancer program that included the NCI, other research institutes and federal and nonfederal programs; funding to establish 15 new cancer research centers and local control programs; and an international cancer research databank. It also provided the director with direct access to the President of the United States and empowered the agency to make long-term public investments to advance cancer control and care delivery research throughout the cancer care continuum.
The increased funding for the NCI amounted to nearly $700 million during the first 7 years following implementation of the law5 (currently, the NCI budget is $6.56 billion). It established the first NCI-designated cancer centers, which today number 71, located across 36 states and the District of Columbia, and expanded the National Clinical Trials Network to focus scientific efforts on the understanding, prevention, detection, and treatment of cancer.
“The National Cancer Program created by the National Cancer Act is a very important part of the NCI’s mission,” said Norman E. Sharpless, MD, Director of the NCI. “As former Director of the Lineberger Comprehensive Cancer Center, I consider the establishment of the National Cancer Program to be one of the most important, if not the most important, parts of the National Cancer Act. It distributed cancer research nationally and raised a lot of funding from state and local governments and private philanthropy as well as multiplied the impact of the program tremendously. There really isn’t another program like it within federal research.”
Transforming How to Understand and Manage Cancer
The intense financial investment and focus on cancer research spurred by the legislation led to a seismic shift in fundamental biologic science and an understanding of the complexity of cancer and how it develops; it also fueled breakthroughs in more effective treatments of the disease. Over the past 50 years, pivotal studies conducted by the NCI’s Clinical Trials Network have resulted in the cure of most childhood malignancies; knowledge about how to harness the immune system against cancer, which now includes such immunotherapy approaches as monoclonal antibodies, immune checkpoint inhibitors, cancer vaccines, and cell-based therapies; the development of life-prolonging adjuvant chemotherapy for many solid tumors; and the discovery of technologies for early detection, screening, and prevention. In addition, the National Cancer Act ushered in a new era of patient engagement and advocacy in the development of cancer clinical trials.


“The National Cancer Act is one of the most transformational pieces of legislation to occur in the 20th century. It is that profound,” said Andrew C. von Eschenbach, MD, President of Samaritan Health Initiatives and Adjunct Professor at The University of Texas MD Anderson Cancer Center and former Director of the NCI from 2002 to 2005 and former Commissioner of the U.S. Food and Drug Administration (FDA) from 2005 to 2009. “Beyond the focus and resources it provided to the NCI, the legislation changed the way we were approaching our ability to understand and manage disease.”
Overcoming Challenges
Although the investment made in cancer over the past 5 decades is still paying off, as witnessed by the more than 17 million cancer survivors today—that number is expected to climb to more than 22 million over the next decade, and the 5-year survival rate is expected to increase to 70%6—challenges remain. Despite a total decrease of 31% in overall cancer death rates from 1991 to 2018, including a 2.4% decline from 2017 to 2018,7 cancer is still the second leading cause of death in the United States behind heart disease, the cause of more than 606,000 deaths each year in this country.7 It has surpassed heart disease in some high- and middle-income countries, including Sweden, Canada, Chile, Argentina, Poland, and Turkey,8 and is a leading cause of death worldwide, accounting for nearly10 million deaths in 2020.9 And treatment for many cancers continues to be too painful, too damaging, too ineffective, too expensive, and too inaccessible for many patients.
How might these obstacles be overcome over the next 10, 20, and even 50 years? What advances might be on the horizon to prolong survival—and actually cure more cancers—and improve the lives of all patients with cancer throughout survivorship? Will it ever be possible to eradicate cancer?
In this retrospective of the 50 years of progress since the enactment of the National Cancer Act and a look at what may lie ahead, we asked leaders in a variety of fields of oncology, including genetics and genomics, immunotherapy, health equity, research, survivorship, patient advocacy, and artificial intelligence, to give us their future predictions on cancer care.
Genetics and Genomics
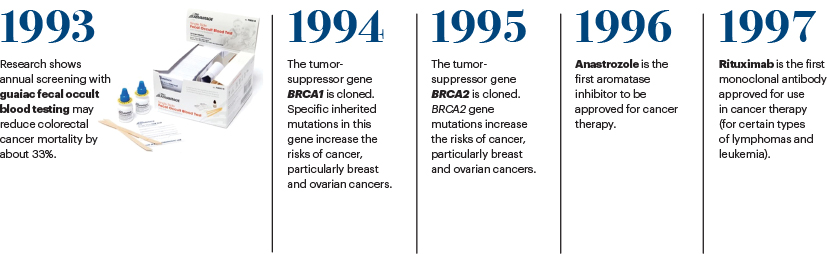
The development in the mid-1990s of a genetic test for the BRCA1/2 gene mutation in breast cancer risk changed the landscape in hereditary breast and ovarian cancer risk assessment, prevention, and treatment. Over the subsequent years, advances in tumor sequencing technology have made it possible to conduct broad genetic testing on individual patients with cancer and identify tumor driver mutations to tailor treatment to improve outcomes.

Olufunmilayo Falusi Olopade, MBBS, OON, FASCO
For more than 2 decades, Olufunmilayo Falusi Olopade, MBBS, OON, FASCO, has pioneered research in cancer genetics, with a special focus on the molecular genetics of breast cancer progression. Her work has led to a greater understanding of the causes of aggressive breast cancer in young women, especially young Black women, and to the integration of genetic predisposition into routine cancer care.
In this interview with The ASCO Post, Dr. Olopade, Walter L. Palmer Distinguished Service Professor and Director of the Center for Global Health at the University of Chicago, describes how genetic screening is becoming integrated into oncology care and how scientific advances will lead to breast cancer prevention and more cures in the coming decades.
Please talk about the widening access to genetic testing in the oncology-care system and how it is leading to more patients with cancer, especially breast and ovarian cancers, being offered genetic screening.
In 1997, I chaired ASCO’s task force on Cancer Genetics Education. The premise was that as oncologists, we know how to break bad news to patients, and we could help high-risk patients understand their risk for developing cancer and how to act on that risk to improve outcomes. Now, in 2021, we are able to realize that dream because ASCO and the cancer community have invested so much in understanding the genetic basis of cancer and using that information for risk stratification.
I trained under cancer genetics pioneer Dr. Janet Rowley, and the tools we had at that time allowed us to look at chromosomes in leukemia and lymphoma, but we didn’t have the ability to study chromosomes in solid tumors. Now, we can do whole-genome sequencing for half the cost of doing cytogenetic testing in the 1990s, which is a cause for celebration.
The triumph of the National Cancer Act is not only that it has resulted in more effective treatments for cancer, but that it also invested in the training of future oncologists and helped create a new field of cancer genetics. We now have a generation of cancer geneticists like me who are trained to integrate genetics into our practice.
Looking toward the future, it is not sufficient to say to a patient you are at high risk. We must now create a preventive oncology group that is focused on prevention and early detection. In the 1990s, we conducted clinical trials showing that estrogen receptor modulators such as tamoxifen or raloxifene could prevent breast cancer in 50% of the women enrolled in the studies. Today, we are studying how to use science and technology to accelerate the adoption of chemoprevention or lifestyle changes that we all can make to reduce that cancer risk.
How can genetic testing reduce disparities in cancer care?
Genetics is built on the foundation of equity and justice. We have become advocates for patients and have made it clear that being born with a BRCA1 or BRCA2 gene mutation is no reason for discrimination by insurance companies. It is also not a reason for patients to feel stigmatized by society. And because equity and justice are the core principles in everything we do in genetics, we were able to advocate for policies that prohibit genetic discrimination. Once patients trusted us, it was easy to do genetic research in underserved and understudied patient populations.
As part of the value-based projects in health care now, health-care systems and insurance companies strive to keep patients healthy; they want to know who is at high risk for cancer. Prevention is better than cure—and less expensive, too. But who pays for genetic testing if a patient doesn’t have health insurance? How can we help patients
gain access to genetic testing in their communities? Even though we are celebrating 50 years of the National Cancer Act, we cannot claim any victory if a large fraction of the population that should benefit from our discoveries are still left behind.
How do you see the field of genetics progressing over the next 10 to 20 years and beyond? Is the goal for a genetic test to be given to all individuals to learn their risk factors and then try to mitigate those risks?
Yes, absolutely. Now, we realize that we are much more than our genes. We are born with the DNA we have, and there is nothing we can do about that. If we identify a patient with a BRCA mutation, for example, the question is when should we know that information? Before the patient ever gets cancer? There are all types of genetic testing happening right now. Our job is to make sure that we are moving the field forward without doing more harm than good to society.
The challenge for the next decade is to do the research to see how genes are modified by our environment and how to eliminate all forms of racial injustice and bias in medicine and society. When patients are not adequately treated because they don’t have access to high-quality care, is their poor outcome because of their genes? No, it is not.
As we get closer to realizing the goal of precision medicine, we will not be talking about the color of patients’ skin, where they grew up, or whether they are rich or poor. We will be talking about how patients can get the care they need, when they need it, and not have to worry about how to pay for it because we will live in a society where we are all in this together.
Over the next 50 years, will it be possible through genetic testing to prevent breast cancer or make it more treatable and curable?
Absolutely. We already have the tools for risk stratification. We are able to predict a patient’s risk for breast cancer. What has been missing is the convergence of the best tools in science and technology and the adoption of those tools in the health-care system in which we are going from the continuum of preventive strategies to supportive care.
I’m optimistic that with a lot of investment in artificial intelligence and wearable devices, as well as being able to recruit patients for clinical trials more quickly, we are going to eliminate all of the inefficiencies that we know are barriers to accelerating progress in curing more cancers. If we can develop mRNA vaccines against the coronavirus in 1 year, the hope is that the oncology community will also be able to figure out how to develop better drugs for patients at warp speed.
Immunotherapy
The development of cancer immunotherapies over the past decade has transformed the treatment of certain cancers, including advanced melanoma and lung cancer, as well as improved overall survival in select patients with late-stage and refractory cancers. The field of immunotherapy, which includes immune checkpoint inhibitors, adoptive cell therapies, monoclonal antibodies, cancer vaccines, and immune system modulators, has evolved so quickly as a promising way to treat and cure cancer. For 3 years in a row, from 2016 to 2018, ASCO has named a type of immunotherapy its top cancer advance of the year in its Clinical Cancer Advances: Annual Report on Progress Against Cancer.10-12 Immunotherapy has become such an integral part of the cancer treatment arsenal, it is now considered the fourth pillar of cancer therapy, along with surgery, radiotherapy, and chemotherapy.

Antoni Ribas, MD, PhD
The ASCO Post talked with Antoni Ribas, MD, PhD, to learn more about how this increasingly important component of cancer care is leading to better outcomes and even cures in some patients. Dr. Ribas is Professor of Medicine, Surgery, and Molecular and Medical Pharmacology at the University of California Los Angeles, (UCLA); Director of the Tumor Immunology Program at the Jonsson Comprehensive Cancer Center and Director of the Parker Institute for Cancer Immunotherapy Center at UCLA; and Immediate Past President of the American Association for Cancer Research.
Please talk about the progress being made in immunotherapies and the tumor heterogeneity involved in patients’ response.
To give some perspective on the bigger picture, it is obvious that advances over the past 50 years in the fight against cancer have mostly been made in prevention and changes in behavior away from lifestyles that include smoking and lead to cancer and in early detection. The pillars of cancer treatment over those years have been surgery, radiation therapy, and chemotherapies, which have nonspecific activities, or more targeted therapies that block a specific mutation that drives cancer cells and not normal cells.
Then, over the past 20 years, we have had this incredible discovery of targeted therapies; they find a specific target in a cancer that can be matched to a specific drug to kill the cancer. In the past 10 years, we have had the fourth pillar in cancer therapy, which is immunotherapy. Studying the science and understanding the immune system, how T cells recognize cancer, and how those T cells are negatively regulated to avoid overactivity to normal tissues have led to the field of immune checkpoint blockade. This line of thinking of how to take away “the brakes” to the immune system, which garnered the Nobel Prize for Dr. James Allison, who pioneered this work, is now the basis of FDA drug approvals for more than 15 cancer indications. These advances are leading to a realistic potential for some patients with several types of metastatic solid tumors to live a normal life despite a diagnosis with a poor prognosis.
However, checkpoint inhibitors—and other types of immunotherapy, including cell therapies—benefit only a minority of patients with cancers such as melanoma, lung, some lymphomas, and microsatellite-unstable cancers, but not those with many other common cancers. So, we are studying these agents in combination with antigen-based therapies, multitargeted kinases, or chemotherapies and are seeing improved outcomes. And I foresee this trend continuing in the years to come.

How will precision oncology improve over the next 10 to 20 years? Will it be possible to cure more cancers, especially in the advanced-stage setting, or extend survival for more patients with difficult-to-treat cancers?
I think the answer is yes. The first stage of precision oncology has been an incredible success. We now understand which altered mutated proteins, the driver oncogenes, are driving a specific cancer; we can develop specific drugs to block those genes, for example, EGFR inhibitors for non–small cell lung cancer (NSCLC), and the breakthrough drug imatinib, which has improved outcomes for patients with chronic myeloid leukemia. Although currently only a minority of cancers have a driver mutation for which we have a targeted drug, with the recent success of KRAS inhibitors that target KRAS mutations, which are among the most prevalent tumor drivers, we are expanding targeted therapies for difficult-to-treat cancers such as pancreatic cancer.
In the future, we are going to understand there is a certain genetic alteration in a cancer that leads to its vulnerability. There are many genetic alterations that drive cancer, and they may lead to synthetic lethality, a concept clinically validated through the efficacy of PARP inhibitors for BRCA1/2-mutant cancers. It works by detecting a cancer alteration that makes a particular cancer vulnerable to blocking a parallel pathway, leading to cancer cell death. I think the concept of synthetic lethality can be applied to other genetic alterations in cancers that cannot be drugged directly but indirectly by blocking a parallel survival pathway.
Where do you see the greatest areas of research progress in oncology over the coming decades: more effective biomarker-driven treatment approaches that offer increased personalized care for difficult-to-treat cancers, including brain and pancreatic cancers; strategies that are effective in predicting response and resistance to immunotherapies; reductions in adverse consequences of cancer and its treatment as well as improvements in quality of life for patients with cancer; or molecular diagnostics, such as liquid biopsy, for the early detection of cancer?
All of these advances will improve over time. And the amount of knowledge that has been generated makes me think we will continue this fast pace of gaining more knowledge to benefit patients.
I want to touch on liquid biopsies and the potential for early detection of cancer. The data are emerging in which you can find rare genetic signals in blood that can tell us whether a cancer has developed and what its origin is. It’s very exciting. For years, we have known there are cancers of an unknown origin in which tissue biopsy does not tell us where they came from, and it has been difficult to match a specific cancer with a treatment.
It turns out that with the epigenetic marks that can be detected in blood, cancers coming from different organs carry the genetic programs of that organ. For example, a cell from a cancer that started in the nose has a certain set of genes that are nose genes, and they are different from a cell that comes from the liver, which has liver genes but became a cancer. The epigenetic program carried in the cancer can tell us the origin of the cancer and allow us to better treat it if it becomes metastatic. This advance, together with all of the advances of precision oncology, including those in immunotherapy and cell therapy, will help us to get better and better in treating patients with cancer.
What do you envision the cancer landscape to be in 50 years? Will it be possible to eradicate cancer?
We are not going to eradicate the development of cancer because our body and our form of life lead to cancers with time. However, I think we will be able to detect the majority of cancers earlier and treat them more effectively. I assume cancer will become a less relevant disease with time, because we will have better detection diagnostics and more precise treatment. I hope in 50 years, cancer will become a minor health problem.
Achieving Health Equity
Although, overall, cancer mortality has decreased in the United States due to progress in cancer prevention, early detection, and more effective treatments, not every patient is benefiting equally. Racial and ethnic minority populations and other underserved populations experience the greatest health disparities. Collectively, Black individuals have the highest death rate and shortest survival of any racial/ethnic group in the United States for most cancers. Black men also have the highest cancer incidence rate.13
The reasons for disparities in cancer care are complex; they include the social determinants of health and less access to high-quality cancer care, as well as differences in tumor biology of some cancers, including triple-negative breast, colorectal, and prostate cancers, diagnosed in Black patients compared with White patients. Medical racism, lack of diversity in clinical trial research participation, and environmental and lifestyle factors also play a role in cancer disparities.

Brian Rivers, PhD, MPH
The ASCO Post talked with Brian Rivers, PhD, MPH, Professor and Director of the Cancer Health Equity Institute at Morehouse School of Medicine, Atlanta, on how health equity might improve for underserved racial and ethnic patients with cancer over the coming decades.
What progress might be possible over the next 10 to 20 years and beyond to remedy disparities in cancer care?
Over the past decade, there has been a tremendous investment made in research that drove a lot of great discovery in cancer and advanced our understanding about how cancer develops and progresses, and how it is best treated. However, disparities in cancer care remain, even though we are seeing a persistent decline in cancer deaths among all groups for most cancers. For example, Black men are disproportionally impacted by prostate cancer compared with White men, and we see such disparities with other racial and ethnic minorities for a number of other cancers as well, including breast and liver cancers. Understanding why these differences exist is still a work in progress.
We have made great strides regarding cancer biology, but there are limitations to our understanding of the fundamental biologic elements of cancer among diverse populations. We need to increase our understanding of cancer biology in these populations, which have historically not been represented in our biomedical research studies.
Although we still have work to do in this area, we can now apply a lot of what we have learned over the past couple of decades to improve care for underserved populations. I’m excited about how this knowledge will transform health care as well as our biomedical research enterprise in terms of how we conduct research and not work in silos but rather collaboratively with private and public partnerships.
These items are all tenets of precision medicine, and this is the promise that will help us achieve equity for all patients with cancer. The speed of these discoveries will be accelerated, and the accomplishments will be achieved in record time. Just look at the progress we made in just 1 year in the development of a vaccine for the coronavirus and what we have learned about the underpinnings of the genomics of the virus. This progress is not just due to the collaboration among our scientists, but through the collaboration from scientists around the world. I see this same unified global collaboration in cancer research.
We have a lot of work to do, but I’m confident that precision medicine will get us to cancer health equity, ensuring that all patients will be able to get the right treatment at the right time.
Clinical trial participation for adults with cancer is less than 5% and even lower for minority populations. Minority patients often face multiple barriers to trial participation, including the out-of-pocket costs associated with clinical trial enrollment; restrictive study designs and eligibility criteria; lack of functional hospital infrastructure to enroll, manage, and track trial participants; and poor oncologist/patient communication. How can these barriers be overcome?
Overcoming these barriers is instrumental to actualize precision medicine for all individuals. Encouraging minority groups to participate in clinical trials has been at the forefront of conversations recently because of COVID-19, along with concern in terms of inclusion of diverse populations in the vaccine trials to ensure that once the vaccine was developed, it would be efficacious for all individuals.

The vaccine trial designs for COVID-19 intentionally employed strategies to encourage diverse participation. We have to do the same in oncology. The NCI has been a great leader in initiating this process in cancer trials, starting with the implementation of the National Cancer Act of 1971. Fifty years later, we are seeing a more robust federal network for clinical trial participation, such as the NCI’s Community Oncology Research Program, which is a national network of cancer care investigators, providers, academia, and other organizations that care for diverse populations of patients across different health-care systems.
However, as you mentioned, the barriers to accessing clinical trials is multifactorial in scope. There are patient-level barriers and system-level barriers. In this frame of a transformative health-care landscape, we have to be more intentional in terms of our strategy for reaching individuals, giving them the right information, and addressing their concerns. For example, the messaging about clinical trial participation often does not address the issue of trust. Patients want to know that they can trust physicians to be honest, clear, and transparent about what the trial entails; what protections are in place for privacy; and what is the return on the value of participation, not just to the trial participants but to society as a whole.
One potential solution to overcome some of these barriers is to have a patient navigator at the trial site. This person can sit with patients and educate them about the research, addressing issues of trust, privacy, and the value of participation, and help them navigate from the consent process to trial enrollment. Having a more diverse oncology workforce, including a more diverse biomedical workforce, would also be beneficial in increasing minority participation in clinical trials.
Will cancer care be more equitable for all underserved groups over the next 50 years?
I am optimistic that we will see health equity for all patients actualized sooner than we think, perhaps within 10 years, because of all the private- and public-funded science-based initiatives and policies we are already seeing to advance cancer health equity. For example, the NCI’s Center to Reduce Cancer Health Disparities is dedicated to reducing the unequal burden of cancer through a combination of basic and community research, patient education, and outreach to communities suffering from health disparities. How successful these efforts are is dependent on federal funding and making sure that allocations from Congress are sufficient to advance the work in precision medicine and cancer health disparities.
I’m also encouraged to see a greater understanding globally of the genomic underpinnings of cancer and better integration of environmental exposures and lifestyle practices that affect the development of cancer. More than 40% of cancers can be prevented14 if modifiable risk factors, such as smoking, alcohol use, obesity, diet, and physical activity, were addressed. And that percentage may be even higher when coupled with adherence to screening guidelines.

Advances in artificial intelligence and the amount of big data being collected on diverse populations will increase our understanding in a much more rapid way of the genetic diversification of malignant tumors and help us get to personalize cancer care for individual patients. Taken together, these efforts are what drives my hope for an end to cancer disparities in minority patients.
Sustaining Public Support for Cancer Research
The impact of the National Cancer Act of 1971 on the treatment of cancer and the care of patients is profound. The landmark legislation created a new, coordinated approach to advancing cancer research and cemented the country’s commitment to basic research. The scientific advances enabled by the act have resulted in FDA approval of about 100 targeted therapies to treat cancers that harbor a specific genomic alteration,15 often achieving remarkable remissions in difficult-to-treat cancers, including NSCLC and metastatic melanoma, and has led to the promise of personalized medicine for all patients with cancer.
In this interview with The ASCO Post, Norman E. Sharpless, MD, Director of the NCI, discussed the impact of the National Cancer Act on cancer care and how it will be possible to accelerate declines in cancer mortality over the coming decades.
How would you assess the impact of the National Cancer Act on cancer progress and the importance of having a robust and sustained national commitment to conquer cancer?
The National Cancer Act provided the NCI with key capabilities and authorities and a commitment to work in a unified effort in cancer research led by the NCI. And that has been very important in a number of ways. Perhaps the most important achievement of the National Cancer Act was that it made cancer a disease we could talk about openly. Prior to 1971, cancer was a source of shame. After passage of the legislation, cancer could be discussed in the open and with a spirit of hope, and that was really important.

Norman E. Sharpless, MD
The Act established the National Cancer Program, which now includes 71 NCI-designated cancer centers across the United States, which is a successful part of the NCI mission. It created the Surveillance, Epidemiology, and End Results Program (SEER), which is the best database of cancer statistics in the world. The legislation also expanded federal funding for cancer and created what is now known as the Frederick National Laboratory for Cancer Research, which is a federally directed biomedical research facility that has been important to the establishment of The Cancer Genome Atlas and the RAS Initiative.
The progress that we are seeing in cancer, particularly in the accelerating progress we have been seeing in the past 5 years, has built upon this foundation of decades of support started by the National Cancer Act.
What areas of research are the NCI funding that may result in more effective treatments over the coming decades?
The important thing to know about cancer therapeutics is that there will be no silver bullets. At one time, people thought that way, and that was the expectation in the 1970s. What we know now is that cancer is many different diseases with their own causes, pathogeneses, and molecular features, and they require different approaches to prevent and treat them. There are different areas of prognosis and survivorship challenges, so cancer requires the ability to think about a very large number of related, but distinct, diseases.
That kind of thinking, which evolved from thinking of cancer as a monolithic entity or a handful of diseases to the modern view that almost every cancer is unique with a unique host, was real progress. In fact, that might be one of the most important things we learned about cancer in the past few years. What we are seeing now with the application of that paradigm is a situation where cancer has been declining in terms of mortality on the order of 1.5% per year since the early 1990s. The large part of that decline is attributable to tobacco control, but other factors were at work as well. And in the past 5 years, the data from SEER and other cancer mortality data sets are reasonably convincing that the trend has increased. So, the rate of decline has increased. It’s more like 2.4% per year closing in on 2.5% per year.
If you want to talk about ending cancer as we know it, which President Joe Biden has made a commitment to, what you are really talking about is accelerating that rate of decline to a much greater degree, to get it well past 3% or 5%; then, we could see fairly dramatic declines in cancer mortality over years and decades. I do not think that kind of acceleration will be brought about by one single thing. It will require better approaches to screening and prevention. In fact, there are exciting new approaches in screening, including, for example, cell-free DNA liquid biopsy tests, better imaging and machine learning, better prevention efforts, greater achievements in tobacco control, and increased attention to obesity in the United States, which is becoming a leading driver of cancer.
And then there are discoveries and FDA approvals of new therapies. We are closing in on probably 100 drugs approved by the FDA for new molecular entities for cancer in the past 3 to 4 years. One real challenge is how to use them altogether to improve effectiveness. Many of them work better in combination than as single agents, so how do we combine these therapies and then sequence them correctly? Do we give chimeric antigen receptor T-cell therapy first, or do we give immunotherapy first? So, knowing how best to combine therapies in the right sequencing order is emerging as a real challenge, but there is no shortage of new ideas. There are also novel ideas to improve radiation oncology. Both surgery and radiation therapy are older modalities used for a long time in cancer, but they can be improved and mixed with novel therapies in a way that leads to better outcomes for patients.
An important aspect in cancer care to keep in mind is not only are we trying to improve cure rates, but we are also trying to improve long-term disease-free survivorship by using treatments with less toxicity and less long-term morbidity. We want to help patients experience a favorable quality of life and reduce cancer morbidity. Those are the challenges we face going forward if we want to make the kind of progress the present administration is seeking.
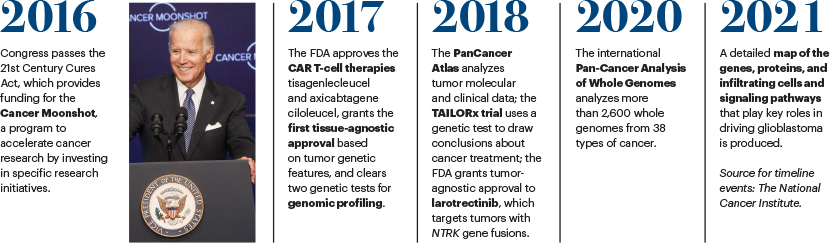
Is the NCI Cancer Moonshot the next national effort that will accelerate cancer progress over the next 50 years?
We are into the fifth year of the Cancer Moonshot Research Initiatives and are off to a good start. We’ve been evaluating the program and have found that the Cancer Moonshot has been supporting more than 240 new research initiatives, and we will see more this year. These initiatives run the gamut of activities from new targets for pediatric cancers, better patient engagement, and germline analysis for families at high risk for cancer to making use of that information clinically. Although it is still too early to tell which research initiative will translate into a change in patient care, the signs so far look promising.
Cancer Survivorship
In 2006, the Institute of Medicine (now the National Academy of Medicine), published its landmark report From Cancer Patient to Cancer Survivor: Lost in Transition.16 The report outlined 10 recommendations to provide coordinated, comprehensive care for cancer survivors. Although the report has been instrumental in improving care for survivors, gaps remain in research, clinical practice, and policy.
In this interview with The ASCO Post, Emily S. Tonorezos, MD, MPH, Director of the Office of Cancer Survivorship at the NCI, talked about how research is leading to improvements in the quality of life for long-term cancer survivors.
In 1996, the NCI established the Office of Cancer Survivorship to support survivorship research and improve clinical care. Please talk about the progress made in research and in the clinical care of cancer survivors over the past 25 years.
It is important to highlight the dual nature of cancer survivorship. Every time we celebrate the success of cancer treatment and the longevity of many survivors, we also have to acknowledge the struggles and challenges many face from the long-term, late effects of cancer and its treatment.

Emily S. Tonorezos, MD, MP
In terms of the progress we’ve made in survivorship research, we always need to start with the progress made in the detection, treatment, and supportive care for cancer. We conduct observational studies to record the late and long-term effects of cancer and then try to develop interventions to care for those survivors. We also look to oncology to adapt or develop new therapies to avoid or reduce late and long-term effects, including infertility, heart disease, secondary neoplasms, and psychosocial issues, as well as the financial toxicity of treatment. That is the nature of our field and the way we make progress in cancer survivorship. And it is the reason we fund a broad array of research to combat both the potential physical ramifications of cancer, such as cardiotoxicity, and the emotional side effects of the disease, such as depression and anxiety.
Based on your research, what is the best way to monitor the general care needs of survivors?
About 2 years ago, scientists here at the NCI, as well as others, developed an evidence-based quality of cancer survivorship care framework.17 That framework sets the stage for health-care delivery research and what clinical care should look like for diverse populations of adult cancer survivors. The framework needs to be tested, but it gives us some structure around how to measure survivorship care quality and how to determine whether we are providing high-quality care.
We have also put out a notice of special interest in receiving research applications focused on identifying important factors for defining risk-stratified survivorship care. This is a personalized approach in which survivors are stratified to distinct care pathways based on the complexity of their needs and the types of providers their care requires.
We have a movement toward patient-centered care, but what does that mean exactly in terms of survivorship care? We have to identify patients’ late effects from cancer treatment and do more to improve the delivery of survivorship care.
What potential progress do you see in cancer survivorship over the next 50 years?
I am very excited about the future. We have our work cut out for us in terms of identifying the side effects of treatment, understanding how to prevent them, supporting primary care, and developing better methods of care coordination. The NCI is investing in the future of survivorship with the Method to Extend Research in Time (MERIT; R-37) Award. The R-37 provides longer-term grant support to early-stage investigators in survivorship care research, and we will reap the benefits of that research for many years, because we will have investigators who stay in survivorship science. I am very encouraged about the progress we will make in survivorship care over the coming decades.
The Impact of Patient Advocacy on Cancer Progress
Arguably, the most important person in propelling the passage of the National Cancer Act of 1971 was Mary Lasker, a philanthropist and longtime activist for cancer research as well as the widow of Chicago advertising executive Albert Lasker, who died of colorectal cancer in 1952. Dubbed the “fairy godmother of medical research” by BusinessWeek magazine, Ms. Lasker used her political skills and contacts to lobby Congress to increase federal expenditures for medical research. Her considerable power and influence helped convene a Senate panel of 26 experts to survey the progress being made in cancer research and make recommendations for how to speed advances, which ultimately resulted in the development of the framework of the National Cancer Act.

Andrew C. von Eschenbach, MD, talked with The ASCO Post about the influencers, including Ms. Lasker, behind the passage of the legislation and the Act’s impact on cancer research today. Dr. von Eschenbach is President of Samaritan Health Initiatives, Adjunct Professor of The University of Texas MD Anderson Cancer Center, and former Director of the NCI and former Commissioner of the FDA.
How influential was Mary Lasker in raising funds for cancer research, increasing the public’s faith in medical science, and promoting the idea that research could find a “cure” for cancer?
This question brings home the convergence of policy and politics and the important role of advocacy in getting legislation passed. There is a lot of political history surrounding how the National Cancer Act came together. There were key people in the Senate, including Ted Kennedy, and Paul Rogers in the House, and people in the Nixon administration working to develop the bill; and in the center of all that political drama was Mary Lasker and leaders from the cancer community, including the American Cancer Society. Mary Lasker was the catalyst dropped into this chemical reaction; by enhancing public awareness and using all of her influence with policymakers who were going to be shaping this legislation, she made cancer a national cause. Advances in cancer research were no longer answers for the very few, but the hope for the many.
None of this kind of research progress happens in Washington without the voice of advocates expressing to policymakers the importance of medical research for cancer, or Alzheimer’s disease, or COVID-19. Mary Lasker was pivotal to cancer research 50 years ago, as are other patient advocates today.
From the passage of the National Cancer Act, the “war on cancer” was controversial. Many leading scientists opposed it, fearing it would take attention away from other research, and it created the unrealistic expectation that a cancer cure was imminent. Since then, other presidents, including President Barack Obama, who launched a national Cancer Moonshot to accelerate cancer research, and now President Joe Biden have vowed to cure cancer. Scientists now know that cancer is hundreds of diseases, with complex causes and triggers, and that there will not be one single cure for all cancer types. Is this latest national effort to “cure” cancer once again raising unrealistic public expectations?
No, it’s not. However, the problem in the beginning was that we didn’t understand cancer. The word “cure” was a word that resonated with people and one they understood. Quite honestly, it was a political word. At the outset, we thought the only option for cancer was to cure it. What has happened over the past 50 years is that we changed the taxonomy of cancer, and now we need to change the terminology. We should never use the word “cure” regarding cancer.

Andrew C. von Eschenbach, MD
What we are actually talking about is eliminating cancer as a threat to someone’s life. We want to eliminate cancer as a cause of suffering as well as premature and unnecessary death. As a result of the National Cancer Act, we have learned how to prevent cancer, how to detect it at its early stage of development, and how to eliminate early-stage cancer, so in that sense, you might want to call it a “cure.” Today, we can also modulate or control cancer so many people live with—but don’t die of—cancer.
Is it rational today, given our 50-year history of cancer progress, to say we can eliminate cancer as a threat to human life? Absolutely, yes, we can, because we now recognize cancer not as an event, but as a process in someone’s life.
How will cancer be perceived over the next 25 to 50 years?
I see the trajectory of cancer taking us to an entirely new place in 20 to 25 years. And we are going to drag all the other life-threatening diseases such as Alzheimer’s disease with us. We have changed the way we think about cancer, the way we understand it, and how to manage it.
“Cure” is a 1971 word. In 2021 and beyond, we are learning how to solve the problem of cancer. We are not finished. A war is not won in one battle, but by a series of battles, and sometimes those battles don’t go well.
We are not finished with cancer, but we are in a much different place than we were 50 years ago. We need another version of the National Cancer Act, but this time, the starting place is light years ahead. In the next 50 years, we will not just be talking about the elimination of disease, we will be talking about the restoration of health. We will apply the tools of cancer research not just to eliminating the cancer cell, but to modulating and enhancing normal cells and restoring health. The door that cancer research has opened will lead us to amazing new places.
How Artificial Intelligence Is Changing Cancer Care
The use of machine learning and artificial intelligence (AI) to diagnose and predict treatment response in patients with cancer has made significant strides over the past decade and will play an increasingly prominent role in oncology over the next decade and beyond. In recognition of technology’s growing influence in cancer care, in 2019, ASCO launched its first-ever international meeting, ASCO Breakthrough: A Global Summit for Oncology Innovators, which was held in Bangkok. The meeting brought together leaders in a variety of fields, including information and computer science, AI, basic and translational research, social media, and biomedical engineering.
A second ASCO Breakthrough meeting is scheduled for August 25–27, 2022, in Yokohama, Japan. In this interview with The ASCO Post, Peter P. Yu, MD, FACP, FASCO, discussed how advances in technology will help improve outcomes for patients with cancer. Dr. Yu is Physician-in-Chief at Hartford HealthCare Cancer Institute, Clinical Member of the Memorial Sloan Kettering Cancer Center, Past President of ASCO, and Chair of Co-Host Committee for ASCO Breakthrough: A Global Summit for Oncology Innovators.

Peter P. Yu, MD, FACP, FASCO
New technologies in cancer care promise to make the detection and treatment of cancer more precise. With the technology developing so quickly, how do you see machine learning evolving over the next decade in the areas of diagnostic imaging solutions, genomics, prognostic prediction, and precision medicine?
Given the pace of technology development, 10 years is a long runway, and some breakthroughs are on the cusp of clinical deployment, including advances in image-recognition technology applicable in radiology. For example, volumetric measurements of a cancer would have tremendous impact on the assessment of cancer response to therapy when compared with current single-dimension measures of cancer size.
We often think of cancers as being nice round spheres, but in reality, they are irregular. It can be challenging for radiologists to determine the borders of a lesion, let alone calculate the volume of an irregular object. With three-dimensional imaging, we will be able to more precisely map the volume of a cancer cell and do it automatically.
Another example is the application of AI to help distinguish benign lesions from malignant ones on cancer screening studies, thereby potentially reducing unnecessary biopsies. The migration from film to digital capture, increase in computational processing power, and more advanced nonlinear machine learning methods, such as convolutional neural networks, allow more accurate assessments of a radiologic findings of a cancer potential. We are ready to start clinical studies to explore clinical utility.
A bit farther down the road, but achievable within the next 5 years, will be the application of AI in pathology to better understand the biology of cancer, the tumor microenvironment, and the interaction between cancer and stroma. Quantifiable assessment of cellular and subcellular anatomic pathology combined with genomic data from clinical pathology will provide insight into genotype-phenotype correlations, leading to improved predictions of cancer behavior in patients.
Today, a pathologist examining a slide has a qualitative impression of vascularity, tumor invasiveness, and immune effector cell response. A quantified assessment of cancer biology may lead to better prediction of response to antiangiogenesis and immune-stimulatory therapies or otherwise provide insight into how cancer biology drives clinical decision-making.
For many years now, military flight missions follow preprogrammed routes using instructions loaded into the flight computer and terrain matching localization. Self-driving cars may be a reality in 5 years. Looking ahead to the 5- to 10-year timeframe, one could conceive that preoperative imaging will enhance robotic surgery with preoperative and interoperative guidance on surgical approaches.
With enough data about the location of a tumor and the proximity of major blood vessels and nerves, surgeons can preplan their operative approach to avoid damaging biostructures while gaining access to the tumor bed and excise the tumor more precisely—a kind of augmented-reality approach to surgery.
Beyond imaging, advances in organoid technology look promising. Three-dimensional printing is being used to construct anatomic representations of organs, such as alveoli, which form a lattice upon which biologic models can be built. Using stem cell technology, endothelial cells to line three-dimensional constructed vascular channels, epithelial cells to line alveoli, as well as fibroblasts and immune cells to populate stroma and then overlaying cancer cell lines, may allow us to study and manipulate the tumor microenvironment. The human microbiome adds another level of complexity as we begin to think beyond the cancer cells and consider the patient-host as the universe within which the cancer exists and where bacteria alter host metabolism, immune response, and biology.
This approach will drive an evolution of our thinking from genetic mutations and gene expression to metabolism as represented by protein function, chemistry, and energy metabolism, creating new targets for drug development.
Will this technology lead to more cancer cures? Will it be possible to eradicate cancer over the next 5 decades?
The blending of imaging, genomic, and clinical data will provide us with a more comprehensive understanding at the patient level of that individual’s cancer and help clinicians make treatment decisions tailored to that patient’s cancer. Advances in acquisition, representation, and storage of an increasing diversity of data; a greater understanding of cancer biology; and increasing computational power to analyze and model cancer treatments will result in more cures.
I don’t know whether we will be able to eradicate all cancers 50 years from now, but we will have the capacity to eradicate most cancers. By that time, the barriers will be less about technology and more about societal values and priorities. Will we have health equity, so cancer cures are available to all? Will we manage our environment to promote health and avoid disease? We don’t know the answers to these questions.
In the coming decades, climate change will become an increasingly bigger global problem. Failure to address this and other societal challenges will mean fewer resources for scientific research and health care as well as exacerbation of the socioeconomic inequality present globally. We have to ask ourselves, how will we as physicians and scientists engage in these societal issues while there is still time to avert disaster?
If we don’t address these issues now, technology will not save us. One of the downsides of technology is our high expectation that we will be able to deliver miracles all the time, but technology has its limits. ASCO’s mission statement was recently revised to “Conquering cancer through research, education, and promotion of the highest quality, equitable patient care.” Technology must be combined with resources to deliver advances in cancer care to the doorstep of every patient across the world. If we as a global community can come together on this, I believe that over the next 50 years, ASCO’s aspirational vision of “A world where cancer is prevented or cured, and every survivor is healthy” will be realized.
DISCLOSURE: Dr. Olopade is co-founder of CancerIQ and an advisor for Tempus and 54gene. Dr. Ribas has received honoraria from consulting with Amgen, Bristol Myers Squibb, Chugai, Genentech, Merck, Novartis, Roche, Sanofi, and Vedanta; has been a member of the scientific advisory board and holds stock in Advaxis, Apricity, Arcus, Compugen, CytomX, Five Prime, Highlight, ImaginAb, Isoplexis, Kalthera, Kite-Gilead, Merus, PACT Pharma, RAPT, Rgenix, and Tango; and has received research funding from Agilent and Bristol Myers Squibb through Stand Up to Cancer. Dr. von Eschenbach is a Board Director for Celularity, Bausch Health Companies, Wren Therapeutics, Radius, Prostate Cancer Foundation, Reagan Udall Foundation of the FDA; a consultant for the Milken Institute, Bipartisan Policy Center, Chugai Pharmaceuticals International Advisory Council; and an advisor for Datavant and Target Pharma Solutions/Norwest Ventures. Dr. Rivers, Dr. Sharpless, Dr. Tonorezos, and Dr. Yu reported no conflicts of interest.
REFERENCES
1. American Cancer Society: Advancement of Cancer Survivorship. Available at www.cancer.org/cancer/cancer-basics/history-of-cancer/cancer-survivorship.html. Accessed April 30, 2021.
2. Parikh RB, Kirch RA, Brawley OW: Advancing a quality-of-life agenda in cancer advocacy: Beyond the war metaphor. JAMA Oncol 1:423-424, 2015.
3. Figg WD, Smith EK, Price DK, et al: Disclosing a diagnosis of cancer: Where and how does it occur? J Clin Oncol 28:3630-3635, 2010.
4. National Cancer Institute: Cancer Facts & the War on Cancer. Available at https://training.seer.cancer.gov/disease/war/. Accessed April 30, 2021.
5. Kalberer Jr JT, Newell Jr GR: Funding impact of the National Cancer Act and beyond. Cancer Res 39:4274-4284, 1979.
6. National Cancer Institute: Cancer Statistics. Available at www.cancer.gov/about-cancer/understanding/statistics. Accessed April 30, 2021.
7. American Cancer Society: Fact & Figures 2021 Reports Another Record-Breaking 1-Year Drop in Cancer Deaths. Available at www.cancer.org/latest-news/facts-and-figures-2021.html. Accessed April 30, 2021.
8. Dagenais GR, Leong DP, Rangarajan S, et al: Variations in common diseases, hospital admissions, and deaths in middle-aged adults in 21 countries from five continents (PURE): A prospective cohort study. Lancet 395:785-794, 2020.
9. World Health Organization: Cancer. Available at www.who.int/news-room/fact-sheets/detail/cancer. Accessed April 30, 2021.
10. ASCO Connection: ASCO Names Immunotherapy as Cancer Advance of the Year, February 4, 2016. Available at https://connection.asco.org/magazine/features/asco-names-immunotherapy-cancer-advance-year. Accessed April 30, 2021.
11. ASCO Connection: ASCO Names Immunotherapy 2.0 as Cancer Advance of the Year in Latest Clinical Cancer Advances Report, February 1, 2017. Available at https://connection.asco.org/magazine/society-member-news/asco-names-immunotherapy-20-cancer-advance-year-latest-clinical-cancer. Accessed April 30, 2021.
12. ASCO Connection: ASCO Names Adoptive Cell Immunotherapy as Cancer Advance of the Year, January 30, 2018. Available at https://connection.asco.org/magazine/society-member-news/asco-names-adoptive-cell-immunotherapy-cancer-advance. Accessed April 30, 2021.
13. American Cancer Society: Cancer Facts & Figures for African Americans 2019–2021. Available at www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/cancer-facts-and-figures-for-african-americans/cancer-facts-and-figures-for-african-americans-2019-2021.pdf. Accessed April 30, 2021.
14. American Cancer Society: More Than 4 in 10 Cancers and Cancer Deaths Linked to Modifiable Risk Factors. Available at www.cancer.org/latest-news/more-than-4-in-10-cancers-and-cancer-deaths-linked-to-modifiable-risk-factors.html. Accessed April 30, 2021.
15. National Cancer Institute: Targeted Cancer Therapies. Available at www.cancer.gov/about-cancer/treatment/types/targeted-therapies/targeted-therapies-fact-sheet#what-targeted-therapies-have-been-approved-for-specific-types-of-cancer. Accessed April 30, 2021.
16. The National Academies of Sciences Engineering Medicine: From Cancer Patient to Cancer Survivor: Lost in Transition (2006). Available at www.nap.edu/catalog/11468/from-cancer-patient-to-cancer-survivor-lost-in-transition. Accessed April 30, 2021.
17. Nekhlyudov L, Mollica MA, Jacobsen PB, et al: Developing a quality of cancer survivorship care framework: Implications for clinical care, research, and policy. J Natl Cancer Inst 111:1120-1130, 2019.

