For recurrent, previously irradiated brain tumors, innovative treatment with surgically targeted brachytherapy yielded good local control and overall survival, as compared to historic controls, neurosurgeons reported at the 2019 Annual Scientific Meeting of the American Association of Neurological Surgeons (AANS).1

This new technology is fundamentally different from the older brachytherapy in both the isotope and the spacing, and this is what has given us our favorable results across a range of tumor histologies.— Peter Nakaji, MD
Tweet this quote
“This treatment could expand the therapeutic options for this difficult cohort of patients,” said Peter Nakaji, MD, of Barrow Neurological Institute in Phoenix. Dr. Nakaji’s research was the winner of the Mahaley Clinical Research Award at the meeting.
In July 2018, the U.S. Food and Drug Administration granted approval for the novel brachytherapy technology, known as GammaTile Therapy, for the treatment of recurrent brain tumors. The device incorporates proprietary cesium (Cs)-131 seeds within customizable collagen-based carriers and delivers 60 to 80 Gy 5 mm deep to the operative bed surface over about 6 weeks.
New Modalities Needed
Resection alone is typically not sufficient for recurrent brain tumors, and repeat adjuvant external-beam radiation therapy (EBRT) has substantial drawbacks. “We can use EBRT a second time, but more and more we are seeing the downside of this, in terms of functional outcomes and quality of life,” he noted.
Traditional brachytherapy is an established technique for treating many kinds of solid tumors, but its benefit in central nervous system tumors has not been proven. Surgically guided collagen tile brachytherapy using the Cs-131 isotope may improve upon current radiotherapy outcomes, including brachytherapy. Targeted reirradiation using seeds is safer, with less impact on quality of life, he maintained.
Tile Brachytherapy Coats the Tumor Bed
The brachytherapy modality achieves seed spacing by imbedding the source in a collagen carrier, which creates a tight conformal distribution and keeps the seeds in place. The tumor bed is coated with these hand-assembled biocompatible collagen tiles, which deliver a high dose of radiation mostly within the first 2 weeks.
TARGETED TILE BRACHYTHERAPY
- The U.S. Food and Drug Administration has approved a novel brachytherapy technology for the treatment of recurrent brain tumors. The new device incorporates proprietary cesium-131 seeds within customizable collagen-based carriers and delivers radiation into the tumor bed.
- In a study of 79 recurrent brain tumors, the median time until local recurrence was 12 months for high-grade gliomas and 48.5 months for meningiomas.
- Time to treatment failure was as good as, or somewhat better than, that achieved with the patients’ previous line of therapy.
The new technique differs from a similar older modality in that it uses Cs-131 as the isotope, a compound with a shorter half-life than iodine-125. The latter isotope was previously studied but was associated with the need for reoperation and/or the development of radiation necrosis.
“This new technology is fundamentally different from the older brachytherapy in both the isotope and the spacing, and this is what has given us our favorable results across a range of tumor histologies,” Dr. Nakaji said.
Treatment of 79 Tumors
The single-arm study included 74 patients (median age = 61 years) with 79 tumors, all treated with maximum safe resection and tile brachytherapy. After completing resection, the neurosurgeon lined the tumor cavity with the tiles in a procedure that takes only about 5 minutes. No additional local therapy was given unless there was disease progression.
The 79 recurrent tumors included 40 high-grade gliomas (grade 3 in 10 patients, grade 4 in 30); 23 meningiomas (grade 1 in 1 patient, grade 2 in 20, grade 3 in 2); 12 metastases; and 4 “other” lesions. Patients averaged two prior same-site surgeries (range = 0–4) and had received a median prior EBRT dose of 70 Gy.
“These truly were patients in need of salvage therapy. They had had multiple rounds of surgery,” he noted.
Key Outcomes
After a median follow-up of 13.4 months (range = 1–54.6 months), the median time for local control was 12.0 months for high-grade gliomas and 48.5 months for meningiomas. For patients with metastasis, the median time to local control was not reached. Median overall survival was 12.0 months, 49.2 months, and 12 months in the three groups, respectively.
“Outcomes with meningiomas were even more striking than for gliomas, with a much more prolonged time to recurrence. And for metastatic tumors, when patients were treated with tile brachytherapy, they did not have a local recurrence before dying of their disease,” he commented.
Time to recurrence was also generally longer after brachytherapy than after a previous, traditional line of treatment, as shown for the 40 patients with high-grade glioma in Table 1.
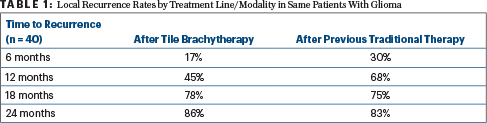
“We compared patients with themselves, looking at their time to failure on previous therapy. We all expect that the next disease progression will be faster, not slower, than before. But we saw, in fact, that even after an average of two resections, patients were getting 12 months of local control. We were quite gratified by this outcome,” Dr. Nakaji said.
Adverse surgical events in 79 cases included wound infection in two patients (2.5%), dural closure breakdown in two patients (2.5%), and procedure-related hematoma in one patient (1.3%). Symptomatic radiation brain changes occurred in six cases (7.6%), all treated medically.
“We had a few adverse events but saw a low amount of wound infection and breakdown of the tumor beds,” he said. “We did see radiation treatment changes or necrosis in six patients, but all were treated medically.”
Dr. Nakaji foresees the new modality fitting well into the treatment paradigm for patients lacking better options at this point. In addition, he hopes to evaluate it sooner in the disease course.
“Say we could resect a new tumor, and instead of standard radiation, we hit it with this right away. Would this slow down the tumor more?” he asked. “It may be that early on is the best time, when there are fewer malignant cells. We know there are cells that reside further away from the tumor, so this won’t be the answer for all patients, but almost all recurrences are local, and if we can control those cases, we may be able to push back the time horizon.”
Randomized Trial Needed
Daniel Orringer, MD, Assistant Professor of Neurosurgery at the University of Michigan, Ann Arbor, said he sees potential

The ideal way to evaluate this is in a head-to-head randomized trial. Once we get those data, we would be in a better position to evaluate its efficacy.— Daniel Orringer, MD
Tweet this quote
benefits to tile brachytherapy in brain tumors. “The current practice with radiation therapy requires patients to go into the hospital or [radiation] center 5 days a week for 6 weeks, and this has an impact on quality of life. Certainly, eliminating 6 weeks of going in for treatment could be beneficial,” he pointed out.
But the approach needs to be validated, if possible, by comparing tile brachytherapy to conventional fractionated radiotherapy, he emphasized. “The ideal way to evaluate this is in a head-to-head randomized trial. Once we get those data, we would be in a better position to evaluate its efficacy,” Dr. Orringer said. ■
DISCLOSURE: Dr. Nakaji holds stock in GT Medical, which is commercializing the treatment discussed in this presentation. Dr. Orringer reported no conflicts of interest.
REFERENCE
1. Nakaji P, Youssef E, Dardis C, et al: Surgically targeted radiation therapy: A prospective trial in 79 recurrent, previously irradiated intracranial neoplasms. 2019 American Association of Neurological Surgeons Annual Scientific Meeting. Abstract 207. Presented April 16, 2019.

