
Rana R. McKay, MD
THE FRONT-LINE systemic treatment landscape for metastatic renal cell carcinoma has undergone tremendous movement over the past several years. A better understanding of the current management paradigm for therapy-naive patients warrants a reflection of historic landmark clinical trials that have changed the management of patients with advanced disease. In 2006, the vascular endothelial growth factor (VEGF) receptor inhibitor sunitinib was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA). The approvals followed the results of a phase III study demonstrating superior progression-free survival and objective responses when compared with interferon-alfa. Since that time, sunitinib has been a standard-of-care, first-line treatment for patients with advanced renal cell carcinoma. Until 2018, no agent or combination was able to demonstrate superior efficacy over sunitinib.
Within the past year, a major shift in the front-line space began to unfold with the FDA and EMA approval of the combination of nivolumab plus ipilimumab for patients with previously untreated intermediate- and poor-risk advanced renal cell carcinoma. In a large, phase III trial (CheckMate 214),1 nivolumab and ipilimumab demonstrated improved overall survival and objective responses over sunitinib. For the first time in over a decade, the combination of nivolumab plus ipilimumab has challenged VEGF monotherapy as a new standard of care for patients with untreated intermediate- and poor-risk advanced renal cell carcinoma.
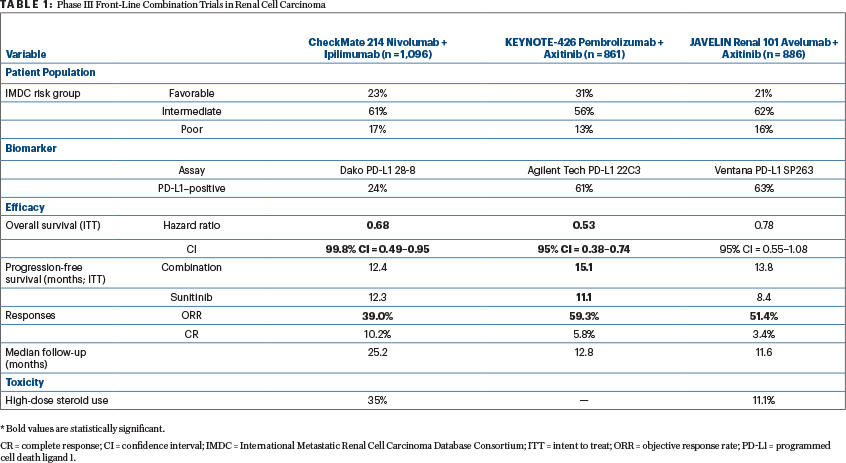
Since the approval of nivolumab plus ipilimumab, data from two phase III trials—reviewed herein—are likely to expand the front-line treatment options for patients with advanced renal cell carcinoma have been reported (Table 1).
JAVELIN Renal 101 evaluated the combination of the programmed cell death ligand 1 (PD-L1) monoclonal antibody avelumab and the VEGF receptor tyrosine kinase inhibitor axitinib initially approved as second-line renal cell carcinoma therapy in 2013.2 KEYNOTE-426 investigated the programmed cell death protein 1 (PD-1) monoclonal antibody pembrolizumab, combined with axitinib. In both studies, the comparator arm was the standard-of-care sunitinib.3
Review of KEYNOTE-426 and JAVELIN Renal 101
IN DISCUSSING these two studies, it is important to focus on key differences and similarities including: (1) the patient population; (2) the primary and secondary efficacy endpoints; (3) the biomarker definition; and (4) the safety profile.
The two studies enrolled different populations of patients. JAVELIN Renal 101 enrolled 886 patients of whom 21.4% (n = 190/886) had favorable-risk disease by International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) criteria. In KEYNOTE-426, a higher percentage of patients had favorable-risk disease (31.2%, n = 269/861), as reflected by the longer progression-free survival in the sunitinib control arm (11.1 months). This finding is contrasted with the progression-free survival of 8.4 months in the sunitinib control arm of JAVELIN Renal 101.
The two studies had differing primary objectives. In JAVELIN Renal 101, the initial primary objective was progression-free survival irrespective of PD-L1 expression; however, following the results of the phase Ib trial of avelumab plus axitinib, the primary objective was amended to either superior progression-free or overall survival in patients with PD-L1–positive tumors. The trial demonstrated that among patients with PD-L1–positive tumors, progression-free survival was prolonged with avelumab plus axitinib compared with sunitinib (13.8 months vs 7.2 months; stratified hazard ratio (HR) = 0.61, 95% confidence interval [CI] = 0.47–0.79; P < .001). This improvement was also demonstrated in the overall patient population. Overall survival data are still maturing, with deaths observed in 13.7% of patients treated with avelumab plus axitinib and 15.2% of patients treated with sunitinib (stratified HR = 0.82, 95% CI = 0.53–1.28; P = .38) with PD-L1–positive tumors.
“Until 2018, no single agent or combination was able to demonstrate superior efficacy over sunitinib [for advanced renal cell carcinoma].”— Rana R. McKay, MD
Tweet this quote
In KEYNOTE-426, there were dual primary endpoints of progression-free and overall survival, irrespective of PD-L1 status. Although the median overall survival was not reached in either group, the estimated percentage of patients alive at 12 months was 89.9% in the pembrolizumab plus axitinib group and 78.3% in the sunitinib group, resulting in a 47% lower risk of death with the combination compared with sunitinib (HR = 0.43, 95% CI = 0.38–0.74). Additionally, progression-free survival was prolonged with pembrolizumab plus axitinib compared with sunitinib (15.1 vs 11.1 months; HR = 0.69, 95% CI = 0.57–0.84; P < .001).
Objective responses occurred in more than half of patients treated with avelumab plus axitinib compared with sunitinib (51.4% vs 25.7%) and pembrolizumab and axitinib compared with sunitinib (59.3% vs 35.7%). Additionally, responses occurred early, with a median time to response of 1.6 months in PD-L1–positive patients receiving avelumab plus axitinib, 2.6 months in the overall population receiving avelumab plus axitinib, and 2.8 months in patients receiving pembrolizumab plus axitinib (imaging timepoints differed between the two studies).
As we think of relevant endpoints in immunotherapy trials, it is worthwhile to highlight the complete response rates in these studies and contrast them with the complete response rate observed with nivolumab plus ipilimumab. The complete response rate was 4.4% in PD-L1–positive patients receiving avelumab plus axitinib and 3.4% in the overall population receiving avelumab plus axitinib. In KEYNOTE-426, the complete response rate was 5.8% in patients receiving pembrolizumab plus axitinib compared with 1.9% with sunitinib. Additionally, KEYNOTE-426 allowed investigators to discontinue treatment with pembrolizumab once a complete response was achieved. In intermediate- and poor-risk patients treated with nivolumab plus ipilimumab, the complete response rate was 9%, and in favorable-risk patients, it was 8%.
“Reconciliation of the optimal assay and definition of PD‑L1 positivity is necessary to understand the significance of PD-L1 expression clinically.”— Rana R. McKay, MD
Tweet this quote
PD-L1 Expression
CURRENTLY IN renal cell carcinoma, PD-L1 expression has been prognostic of worse outcomes; however, it has not been used as a predictive biomarker of response to immunotherapy. Both JAVELIN Renal 101 and KEYNOTE-426 performed PD-L1 staining on tumor tissue, although the assays used and the definitions of positivity differed between these two studies. JAVELIN Renal 101 used the Ventana PD-L1 SP263 assay and defined positivity as at least 1% of immune cells staining positive; KEYNOTE-426 used the PD-L1 IHC 22C3 pharmDx assay and defined positivity as a combined positive score of at least 1% (PD-L1–positive tumor cells/lymphocytes/macrophages divided by the total number of cells).
The majority of patients were PD-L1–positive in both these studies (63.2%, n = 560/886 for JAVELIN Renal 101; 60.5%, n = 497/822 for KEYNOTE-426). These results are in contrast to the CheckMate 214 data of nivolumab plus ipilimumab in which 24% of patients (n = 240/1,002) were PD-L1–positive. Additional studies are warranted to identify predictive biomarkers of response to immunotherapy in renal cell carcinoma. Furthermore, reconciliation of the optimal assay and definition of PD-L1 positivity is necessary to understand the significance of PD-L1 expression clinically.
Safety Profiles
LASTLY, IT IS valuable to emphasize the safety profiles of these combinations compared with sunitinib. Avelumab is administered every 2 weeks and requires premedication with an antihistamine and acetaminophen. All-grade infusion-related reactions were observed in 12.2% of patients receiving avelumab plus axitinib. Unlike avelumab, pembrolizumab is administered every 3 weeks and does not require premedications.
In JAVELIN Renal 101, 9.0% of patients experienced grade ≥ 3 immune-related adverse events and 11.1% required high-dose glucocorticoids for management of immune-related adverse events related to avelumab plus axitinib. In KEYNOTE-426, grade 3, 4, and 5 adverse events of interest occurred in 8.4%, 1.6%, and 0.7% of patients treated with pembrolizumab plus axitinib. The rates of grade ≥ 3 adverse events were lower in these combination studies than those observed with nivolumab plus ipilimumab (46% of patients with grade ≥ 3 adverse events; 35% of patients required high-dose glucocorticoids for adverse event management).
JAVELIN Renal 101 and KEYNOTE-426 are landmark studies that will challenge the front-line treatment paradigm in the management of advanced renal cell carcinoma. Despite the observed efficacy of these combinatorial studies, the optimal combination that will be used in the future is still undefined. Additionally, many questions remain unanswered, including selecting patients, enhancing long-term durability of response, and balancing risks of toxicity. Furthermore, as the front-line space is evolving, new questions arise regarding the optimal second-line treatment strategies and appropriate treatment sequences. ■
DISCLOSURE: Dr. McKay has served as an advisor/consultant for Janssen, Novartis, Bristol-Myers Squibb/Pfizer, Tempus, and Exelixis, and has received institutional research funding from Pfizer and Bayer.
REFERENCES
1. Motzer RJ, Tannir NM, McDermott DF, et al: Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277- 1290, 2018.
2. Motzer RJ, Penkov K, Haanen J, et al: Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1103-1115, 2019.
3. Rini BI, Plimack ER, Stus V, et al: Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 380:1116-1127, 2019.


