
Syed Abutalib, MD
Here are several abstracts selected from the proceedings of the 2016 American Society of Hematology (ASH) Annual Meeting & Exposition, highlighting newer therapeutics in smoldering and relapsed and/or refractory multiple myeloma. For the full details of these study abstracts, visit http://www.bloodjournal.org/content/128/22.
Smoldering Multiple Myeloma
Abstract 3339: Sustained high rates of complete response and minimal residual disease (MRD) negativity after 8 cycles of carfilzomib [Kyprolis], lenalidomide [Revlimid], and dexamethasone followed by 2 years of lenalidomide maintenance (CRd-R) in patients (n = 18) with high-risk (defined by International Myeloma Working Group [IMWG] 2010 criteria, Mayo, or PETHEMA models) smoldering multiple myeloma: Updated results of clinical and correlative phase II study1
Study Endpoints: The primary objective was overall response rate, followed by secondary objectives of progression-free survival and duration of response, which were assessed after every cycle of induction and every 90 days during maintenance. Correlative studies, including assessment of MRD by multicolor flow cytometry (bone marrow aspirate; 10-5 sensitivity) as defined by updated 2016 IMWG response criteria, were performed after 8 cycles of induction and 1 and 2 years of maintenance lenalidomide.
Abstract Conclusion: Early treatment of high-risk smoldering multiple myeloma with modern CRd-R combination therapy with by-default-delayed autologous hematopoietic cell transplant (auto-HCT) resulted in high rates of complete response and MRDneg complete response after 8 cycles of CRd. Following 2 years of additional lenalidomide maintenance therapy, the complete response and sustained MRDneg complete response rates were 88.9% and 69.2%, respectively. Duration of response and progression-free survival at 36 months were 94%, and overall survival was 100%, with a median follow-up duration of 31 months. Significant serious adverse events included congestive heart failure, which occurred in one patient.
Clinical Implications: Given the significant risk of progression to symptomatic multiple myeloma and associated life-limiting end-organ damage, early intervention for patients with high-risk smoldering multiple myeloma with triplet-based therapies may be warranted. At present, there are no clinical implications of these data; however, early treatment may decrease the risk of disease progression and prolong survival specifically in these patients. The long-term follow-up results of this and other such studies in these patients will have important clinical implications.
Abstract 976: Phase II trial of combination of elotuzumab [Empliciti], lenalidomide, and dexamethasone in patients (n = 39) with high-risk smoldering multiple myeloma2
Study Endpoint: The rate of progression to symptomatic multiple myeloma
Abstract Conclusion: The median number of cycles completed is 6 (range, 1–19). Therapy-related grade 3 toxicities included hypophosphatemia (23%), neutropenia (8%), infection (8%), anemia (3%), pulmonary embolism (3%), rash (3%), and diarrhea (3%). No related grade 4 or 5 toxicities occurred at the time of publication. CD34-positive cell collection was successful in all patients at the time of publication. Unrelated toxicities included one instance of grade 4 prolonged QTc Interval. Of the 34 evaluable patients enrolled to both arms of the study, the clinical benefit rate is 97%. The overall response rate is 71%, including 9 very good partial responses (26%) and 15 partial responses (44%). The very good partial responses cases were under evaluation of possible complete responses due to the potential interference of elotuzumab with immunoelectrophoresis. At the time of publication, no patients had progressed to active multiple myeloma during or after protocol therapy.
Clinical Implications: The high response rates among this patient population, who would otherwise remain untreated, should be viewed carefully with associated intervention-related toxicity. Longer follow-up is required before definitive conclusions can be drawn on the benefits of such intervention in patients with high-risk smoldering multiple myeloma.
Relapsed and/or Refractory Multiple Myeloma
Abstract 1145: A multicenter, open-label phase I/II study of weekly carfilzomib, pomalidomide [Pomalyst], and dexamethasone (CPD) in patients with relapsed and/or refractory multiple myeloma (n = 52 in phase I and n = 42 in phase II)3
Study Endpoint: The primary objective of the phase I part of the trial was to determine the maximum tolerated dose (MTD) of weekly CPD combination. The primary objective of the phase II part of the trial was to determine the rate of partial response.
Abstract Conclusion: All the dose-limiting toxicities were cardiologic and occurred in patients with a history of cardiac disease. The MTD was established at dose level –1, with carfilzomib at 20/27 mg/m2, pomalidomide at 4 mg, and dexamethasone at 40 mg. Considering both phase I and II portions of the study, the most frequent drug-related, grade ≥ 3 adverse events were hematologic and cardiologic (mainly hypertension). The overall response rate of the phase I/II portions was 58% (30 of 52), including at least very good partial remission in 25% (13 of 52) of patients. With a median follow-up of 10 months, median progression-free survival was 9.5 months, and median overall survival was not reached.
Clinical Implications: This combination was effective in relapsed and/or refractory multiple myeloma. After a median follow-up of 10 months, weekly CRD showed a doubling of the median progression-free survival in comparison with a previously reported regimen of pomalidomide and low-dose dexamethasone (phase III MM-003 trial4; 9.5 vs 4 months respectively), confirming the efficacy of combining a proteasome inhibitor with an immunomodulatory agent.
Abstract 246: Evaluation of MRD in relapsed and/or refractory multiple myeloma patients treated with daratumumab [Darzalex] in combination with lenalidomide plus dexamethasone (POLLUX5) or bortezomib [Velcade] plus dexamethsone (CASTOR6). This is the first comprehensive and prospective study of MRD to date in randomized phase III clinical trials of these patients.7
Study Endpoint: The effect of daratumumab-containing regimens on MRD in relapsed and/or refractory multiple myeloma. MRD was assessed on bone marrow aspirate samples prepared using Ficoll and evaluated by the ClonoSEQTMassay at sensitivities of 0.01% (1 cancer cell/10,000 nucleated cells or 10-4), 0.001% (10-5), and 0.0001% (10-6).
Abstract Conclusion: MRD was assessed in POLLUX (blinded to the treatment group) at the time of suspected complete response and at 3 and 6 months postsuspected complete response for patients who maintained this response. Similarly, in CASTOR, MRD was assessed for patients at the time of suspected complete response (blinded to the treatment group) and at 6 and 12 months after the first dose (at the end and 6 months after the end of bortezomib plus dexamethsone background therapy, respectively). The median duration of follow-up was 13.5 months and 7.4 months in POLLUX and CASTOR, respectively.
The addition of daratumumab resulted in significantly higher MRD-negative rates at all 3 thresholds examined (10-4, 10-5, and 10-6). Additionally, patients who achieved MRD-negative status experienced fewer progression-free survival events compared with MRD-positive patients at a threshold of 10-5 sensitivity. Among patients who achieved at least a complete response, the rate of MRD negativity was at least threefold higher across all sensitivity thresholds in the daratumumab-containing regimen groups compared with cohorts on a two-drug regimen. Interestingly, in MRD-positive patients, progression-free survival was significantly longer in the daratumumab-containing triplet vs doublets.
Clinical Implications: Daratumumab-containing triplet regimens5-7 appear to be superior to nondaratumumab-containing doublets in patients with relapsed and/or refractory multiple myeloma.
Abstract 492: A retrospective analysis of clinical efficacy with daratumumab, pomalidomide, and dexamethasone (DPD) in patients with relapsed and/or refractory multiple myeloma: Utility of retreatment with daratumumab among refractory patients8
Study Endpoint: Overall response rate among patients who are treatment-naive to daratumumab and pomalidomide (cohort 1), refractory to daratumumab or pomalidomide (cohort 2), and refractory to daratumumab and pomalidomide (cohort 3)
Abstract Conclusion: About 97% of the patients were refractory to lenalidomide, 86% were refractory to bortezomib, and 90% had prior HCT. The overall response rates and median progression-free survival are summarized in Table 1.
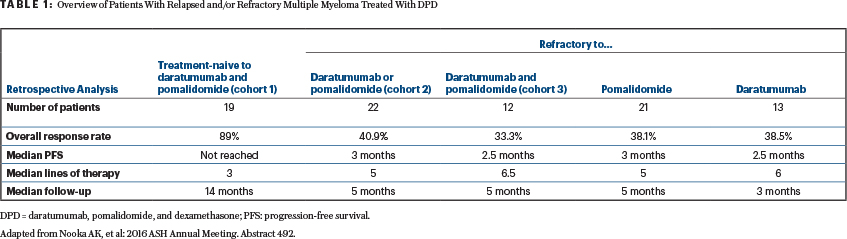
Clinical Implications: Table 1 shows a retrospective provocative data set. Recapturing the responses with DPD in patients who were refractory to both daratumumab and pomalidomide or one of these agents has clinical implications.
Abstract 3311: Safety results of a phase II multicenter, open-label study of pomalidomide plus low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma and renal impairment9
Study Endpoint: The safety profile of pomalidomide plus low-dose dexamethasone in patients with relapsed and/or refractory multiple myeloma and renal impairment, including patients on hemodialysis. The study enrolled three cohorts of patients with differing degrees of renal impairment. Cohort A (n = 30) included patients with moderate renal impairment (eGFR between 30 and 45 mL/min/1.73 m2); cohort B (n = 34) included patients with severe renal impairment (eGFR ≤ 30 mL/min/1.73 m2) not requiring hemodialysis; and cohort C (n = 14) included patients with severe renal impairment requiring hemodialysis.
Abstract Conclusion: All patients received treatment with starting doses of pomalidomide at 4 mg plus dexamethasone at 40 mg (adjusted to 20 mg for those older than age 75). Dose reductions occurred in 12 patients (4, 6, and 2 patients in cohorts A, B, and C, respectively). During the study, 27 patients discontinued treatment due to progressive disease, and 8 patients, due to adverse events in cohorts A, B, and C, respectively. Occurrence of ≥ 1 grade 3 and 4 adverse events was similar between the three cohorts. In cohorts A, B, and C, the occurrence of serious adverse events was 48.3%, 52.9%, and 76.9%, respectively. Rates of asthenia and fatigue were slightly higher in patients on hemodialysis compared with those in cohorts A and B.
Clinical Implications: The results of this study show a similar adverse event profile with pomalidomide and dexamethsone in patients with renal impairment as reported previously in patients without renal insufficiency. Compared with patients not on hemodialysis, slightly more nonhematologic adverse events were reported in patients on hemodialysis.
Abstract 3329: Phase II study of carfilzomib, pegylated liposomal doxorubicin (PLD; 30 mg/m2 on day 8), and dexamethasone in patients (n = 23) with relapsed and/or refractory multiple myeloma10
Study Endpoint: Efficacy of this regimen in relapsed and/or refractory multiple myeloma. Following 6 cycles of combination therapy, PLD was discontinued, and patients were treated with once weekly maintenance therapy with carfilzomib and dexamethsone.
Abstract Conclusion: The overall response rate was 83% (95% confidence interval: 67%–98%), with 48% of the patients achieving a complete or very good partial response. The median estimated event-free survival was 7.4 months at a median follow-up of 10.6 months. Five patients discontinued therapy due to progressive disease; 7 patients, due to toxicity; 1 patient underwent a second transplant; and 10 patients remained on treatment at the time of abstract submission. Grade 3 and 4 nonhematologic toxicity was uncommon. The median number of prior therapies was 2 (range, 1–13).
Clinical Implications: The carfilzomib, PLD, and dexamethasone regimen is well tolerated and appears to be an efficacious strategy in such a poor-risk group of patients with relapsed and/or refractory multiple myeloma. ■
Disclosure: Dr. Abutalib reported no conflicts of interest.
References
1. Kazandjian D, Korde NS, Roschewski M, et al: Sustained high rates of complete response and minimal residual disease negativity after 8 cycles of carfilzomib, lenalidomide, and dexamethasone followed by 2 years of lenalidomide maintenance in patients with high-risk smoldering multiple myeloma: Updated results of clinical and correlative phase 2 study. 2016 ASH Annual Meeting. Abstract 3339.
2. Ghobrial IM, Badros AZ, Vredenburgh JJ, et al: Phase II trial of combination of elotuzumab, lenalidomide, and dexamethasone in high-risk smoldering multiple myeloma. 2016 ASH Annual Meeting. Abstract 976.
3. Bringhen S, Magarotto V, Liberati AM, et al: A multicenter, open label phase I/II study of carfilzomib, pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma patients. 2016 ASH Annual Meeting. Abstract 1145.
4. Weisel K, Dimopoulos M, Song KW, et al: Pomalidomide and low-dose dexamethasone improves health-related quality of life and prolongs time to worsening in relapsed/refractory patients with multiple myeloma enrolled in the MM-003 randomized phase III trial. Clin Lymphoma Myeloma Leuk 15:519-530, 2015.
5. Dimopoulos MA, Oriol A, Nahi H, et al: Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016.
6. Palumbo A, Chanan-Khan A, Weisel K, et al: Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754-676, 2016.
7. Avet-Loiseau H, Casneuf T, Chiu C, et al: Evaluation of minimal residual disease in relapsed/refractory multiple myeloma patients treated with daratumumab in combination with lenalidomide plus dexamethasone or bortezomib plus dexamethsone. 2016 ASH Annual Meeting. Abstract 246.
8. Nooka, AK, Joseph N, Boise LH, et al: Clinical efficacy of daratumumab, pomalidomide and dexamethasone in relapsed, refractory myeloma patients: Utility of retreatment with daratumumab among refractory patients. 2016 ASH Annual Meeting. Abstract 492.
9. Weisel K, Dimopoulos M, van de Donk NWCJ, et al: Safety results of a phase 2 multicenter, open-label study of pomalidomide (CC-4047) plus low-dose dexamethasone in patients with relapsed/refractory multiple myeloma and renal impairment. 2016 ASH Annual Meeting. Abstract 3311.
10. Fiala MA, Keller J, Sekhar J, et al: Phase II study of carfilzomib, pegylated liposomal doxorubicin, and dexamethasone for relapsed or refractory multiple myeloma. 2016 ASH Annual Meeting. Abstract 3329.

