The neurosurgeon is often the gateway provider when patients present with what on magnetic resonance imaging (MRI) appears to be a new glioblastoma. Because histology-based diagnosis is a prerequisite for initiating standard therapy with radiation and chemotherapy, the first question that the neurosurgeon faces is whether to perform a stereotactic biopsy or a craniotomy for resection, which may relieve mass effect and achieve cytoreduction.
The surgeon in question takes into account a variety of factors, including the size of the lesion, its location, the presence of midline shift or other markers of mass effect—each of which may correlate with the patient’s neurologic status or Karnofsky performance status. For decades, Karnofsky performance status has been known to be an independent prognostic indicator of survival for patients with glioblastoma, and, to some extent, a goal of therapy has been to preserve functional status for as long as possible for each patient.
With these issues in mind, the surgeon confronting the case specifically asks, “Is this tumor resectable?” In particular, can enough of the tumor be removed without negatively impacting neurologic status and Karnofsky performance status, so that the patient can enjoy the benefits of decompression and cytoreduction? Related questions include: Are the risks of resection worth the potential benefits, and what tools exist to maximize both the extent and safety of resection?
Glioblastoma Remains Incurable
Glioblastoma (grade IV astrocytoma) remains an incurable malignancy, with an expected median overall survival between 14 and 17 months.1,2 While glioblastomas possess most of the canonical hallmarks of malignancy, part of their resistance to local therapies is likely due to their infiltrative nature. From the time in 1923 when neurosurgeon Walter Dandy attempted to cure glioblastoma by hemispherectomy and failed because of contralateral recurrence in the opposite hemisphere, it has been understood that glioblastoma cells infiltrate significant distances across white matter from radiographically and clinically evident primary masses, and that, therefore, surgery may not be a centrally important component of care.
Despite these facts, glioblastoma today is frequently treated by resection, both at the time of initial presentation and at recurrence. A great deal of effort, technology, and expense is directed toward optimizing craniotomy outcomes, with the parallel and sometimes competing goals of maximizing the extent of removal of the tumor while preserving neurologic function.
Historically, the therapeutic value of aggressive surgical resection of glial tumors has been met with skepticism. For patients with large, symptomatic, and peripherally located tumors, surgical debulking has been pursued for its palliative impact, but its survival benefit has been frequently questioned.
Over the past 10 to 15 years, we have seen advances in radiographic imaging of brain tumors and in adjuncts to surgery, such as increasingly accurate navigation, functional imaging, neurophysiologic mapping techniques, and intraoperative imaging. Similarly, the introduction of temozolomide as an effective chemotherapy in combination with radiation therapy for newly diagnosed patients and the frequent use of bevacizumab (Avastin) for patients with recurrent disease have improved outcomes for glioblastoma patients, with overall survival now between 14 and 18 months.
During the same period, evidence supporting the survival value of upfront aggressive resection of newly diagnosed glioblastoma has accumulated, making craniotomy for these patients the current standard of care. The resection of tumors from the brain, however, can be fraught with neurologic morbidity, negatively impacting quality of life and, ultimately, survival itself. Iatrogenic complications, surgical or otherwise, can have a dramatically negative impact.3
Accordingly, significant investigation and debate have addressed the value of aggressive resection of glioblastoma, with a gross total resection defined by removal of 100% of the gadolinium-enhancing material on MRI. As infiltrating astrocytoma cells invariably exist beyond the gadolinium-enhancing core of the tumor and radical resection is not feasible within the central nervous system, it is not intuitive that “100%” or “gross total” resection is a meaningful goal.
Resectability and Survival
The question of resectability of glioblastoma tumors is a nuanced one with expected variance in opinion across the spectrum of tumors and surgeons alike. Patient-related factors, including age, Karnofsky performance status, and marital status; tumor-associated characteristics, including size, location, and MRI appearance; and provider-related circumstances, including specialist status, volume of practice, training, experience, and incentives may influence rates of resection, and these can be difficult to disentangle outside of the environment of a randomized study.
In a study by Vuorinen et al,4 30 patients aged 65 and older, with presumed newly diagnosed glioblastoma and good performance scores, were randomly assigned to craniotomy vs biopsy. Patients who underwent craniotomy rather than biopsy had significantly improved overall survival and also trended toward longer times to neurologic deterioration. This trial has been criticized because of the specific patient population, the subjects’ relatively poor performance scores, variable postoperative treatments, and small sample size. Also, the authors did not provide information about the extent of resection in patients who underwent craniotomy.
The first decade of the 21st century saw the publication of several retrospective single-center studies and database analyses asserting not only that craniotomy produces a survival benefit in glioblastoma patients, but also that a greater extent of tumor removal is associated with relatively better outcomes. It is generally understood that leaving a significant amount of bulky residual tumor after craniotomy may lead to greater perioperative morbidity and neurologic deterioration, related to cerebral edema or intratumoral hemorrhage.
The general, though not universal, approach has gravitated toward craniotomy in situations when medical and anatomic factors make it feasible. A randomized study examining intentional subtotal resection vs gross total resection would be considered unethical within the field of neuro-oncology, so there will never be class I evidence addressing the survival impact of surgically removing all of the radiographically evident tumor. However, the best current evidence supporting the value of gross total resection of glioblastoma tumors comes from randomized studies examining the value of modern adjuncts that help surgeons intraoperatively differentiate glioblastoma tissue from surrounding normal structures.
Fluorescence-Guided Resection
In 2006, Stummer et al5 published a randomized trial examining the utility of fluorescence-guided resection of glioblastomas vs standard techniques. 5-Aminolevulinic acid (5-ALA) is a nonfluorescent prodrug that leads to accumulation of fluorescent porphyrins within malignant glioma cells, which can then be identified intraoperatively under blue light. 5-ALA can be administered to glioblastoma patients intravenously or orally prior to craniotomy, and early studies confirmed tumor visualization by operating neurosurgeons. The primary endpoints of this multicenter randomized study were the number of patients in each arm without gadolinium-enhancing tumor on immediate postoperative MRI (ie, gross total resection) and progression-free survival.
Enrolled subjects were deemed by study surgeons to have resectable lesions likely to be glioblastoma. The study was terminated at interim analysis because of the significant benefit of fluorescence-guided surgery toward achieving a gross total resection: 65% of patients treated with 5-ALA vs 36% of patients undergoing conventional surgery had no residual contrast-enhancing tumor after craniotomy, independent of age, Karnofsky performance status, or location of the tumor. By multivariate analysis, receipt of 5-ALA and fluorescence-guided surgery was the most important predictive factor. Progression-free survival was better for patients in the 5-ALA group as well: 5.1 vs 3.6 months, independent of Karnofsky performance status and tumor location.
The investigators group-stratified protocol patients by postoperative MRI findings, specifically whether a gross total resection had been achieved. Of note, the average amount of residual contrast remaining was very low in the group of subtotally resected tumors. Overall survival was significantly improved for patients who had undergone gross total resection as opposed to having residual contrast postoperatively (17.9 vs 12.9 months). This finding was maintained on multivariate analysis, which demonstrated that gross total resection, age < 55 years, and Karnofsky performance status > 80 were the most important predictors of overall survival.
This study has been criticized because, among other limitations, the postoperative treatments were not necessarily uniform, though all patients received involved-field radiation. Patients who underwent gross total resection were younger and had less frequent eloquent location of their tumors—stratification by these factors showed that the survival advantage for full resection persists regardless of age or eloquent location of tumor.6
Achieving Total Resection
The 5-ALA studies serve as the current basis for recommending efforts at gross total resection when it can be achieved safely. The use of 5-ALA as an adjunct effectively randomized the patient population into gross totally resected and subtotally resected groups. 5-ALA is not approved by the U.S. Food and Drug Administration for use in patients undergoing surgery and is available only for investigational use.
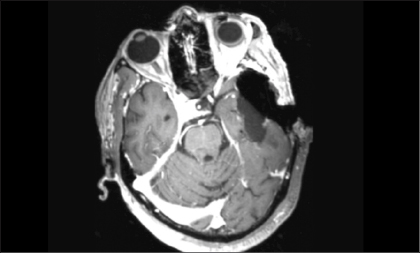
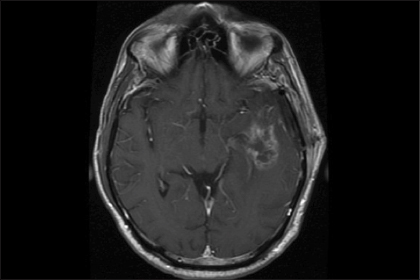
Intraoperative MRI is a more expensive and labor-intensive—but increasingly widespread—alternative adjunct toward achieving total resection of brain tumors (Fig. 1). Similar to the 5-ALA story, a randomized clinical trial examining the use of intraoperative MRI has demonstrated the efficacy of the adjunct in achieving gross total resection and better progression-free survival in patients with glioblastoma.7
Does Subtotal Resection Improve Survival?
An important question that the 5-ALA studies do not address is whether extent of resection is an all-or-none issue. Is all of the impact lost if 95% of the volumetrically measured contrast-enhancing tumor is resected, as opposed to 100%? Two recent retrospective studies suggest that there is graded benefit to larger extents of resection for patients with glioblastoma.
A study by Chaichana et al8 concluded that the minimum extent of resection associated with prolonged survival for patients with newly diagnosed glioblastoma is 70% and that this factor is key in prognosis. For every 5% increment in extent of resection beyond 70%, the hazard ratio decreased by 5.2%.
Sanai et al9 studied 500 consecutive patients who underwent resection of glioblastoma and observed a significant survival advantage for those who had at least 78% of the contrast-enhancing tumor removed. As increasing amounts of tumor were removed, survival improved, even at the upper limits of resection (ie, between 95% and 100%).
Do All Glioblastomas ‘Respond’ to Gross Total Resection?
Although the 5-ALA dataset provides the best contemporary prospective randomized evidence in favor of the survival impact of gross total resection of glioblastoma, the story may continue to evolve. Most of those patients were diagnosed and treated before the regular use of temozolomide chemotherapy, and while the tumors were otherwise fairly well matched, some recently established and impactful molecular features were not studied.
Just as temozolomide chemotherapy is more effective against tumors with a methylated O(6)-methylguanine-DNA-methyltransferase (MGMT) promoter, surgery may prove to be more useful in certain molecularly defined subgroups of glioblastoma. Mutation of the isocitrate dehydrogenase-1 gene (IDH1) is now understood to be a significant positive predictor of survival for patients with glial tumors, both low-grade and malignant. For gadolininum-enhancing malignant astrocytomas, age, Karnofsky performance status, and IDH1 mutation are independently associated not just with survival, but also with the likelihood of achieving a gross total resection.10
This is the first demonstration of a molecular predictor of resectability in brain tumors. Importantly, that molecular predictor is also associated with better survival. In addition, this study reported that, in IDH1-mutated tumors specifically, resection of the nonenhancing components of the tumor was critical for achieving enhanced survival. The relationship between resectability and survival may be more complex than even currently understood and will require continued study.
In Summary
Defining resectability of glioblastoma tumors is complicated; the tumor biology and cerebral anatomy do not accommodate radical resection of tumors. However, gross total resection of the contrast-enhancing portions of these tumors appears to be associated with significantly enhanced survival, and adjunctive measures such as intraoperative MRI and fluorescence-guided surgery may be associated with improved outcomes. Nevertheless, neurologic function should not be compromised by surgical efforts at resection, as the Karnofsky performance scale remains an independent prognostic indicator.
Patients may also derive survival impact from subtotal resection of glioblastoma, with a possible benefit threshold of approximately 70% volumetric resection of the contrast-enhancing portion of the tumor. Further study may define subsets of tumors for which resection is particularly valuable. ■
Disclosure: Dr. Curry reported no potential conflicts of interest.
William T. Curry, Jr, MD, is Director of Neurosurgical Oncology at Massachusetts General Hospital and Associate Professor of Neurosurgery at Harvard Medical School in Boston.
References
1. Stupp R, Mason WP, van den Bent MJ, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987-996, 2005.
2. Gilbert MR, Dignam JJ, Armstrong TS, et al: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370:699-708, 2014.
3. McGirt MJ, Mukherjee D, Chaichana KL, et al: Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery 65:463-469, 2009.
4. Vuorinen V, Hinkka S, Färkkilä M, et al: Debulking or biopsy of malignant glioma in elderly people—a randomised study. Acta Neurochir (Wien) 145:5-10, 2003.
5. Stummer W, Pichlmeier U, Meinel T, et al: Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol 7:392-401, 2006.
6. Stummer W, Reulen HJ, Meinel T, et al: Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery, 62:564-576, 2008.
7. Senft C, Bink A, Franz K, et al: Intraoperative MRI guidance and extent of resection in glioma surgery: A randomised, controlled trial. Lancet Oncol 12:997-1003, 2011.
8. Chaichana KL, Jusue-Torres I, Navarro-Ramirez R, et al: Establishing percent resection and residual volume thresholds affecting survival and recurrence for patients with newly diagnosed intracranial glioblastoma. Neuro Oncol 2014. 16:113-122, 2014.
9. Sanai N, Polley MY, McDermott MW, et al: An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3-8, 2011.
10. Beiko J, Suki D, Hess KR, et al: IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro Oncol 16:81-91, 2014.
Pearls in Neuro-oncology is guest edited by Tracy Batchelor, MD, Director, Division of Neuro-Oncology, Massachusetts General Hospital Cancer Center, and Professor of Neurology, Harvard Medical School, Boston. The series is intended to provide the practicing oncologist with guidance in managing neuro-oncology issues that may present in their patients with cancer.