Non-small cell lung cancer (NSCLC) harboring ALK rearrangement is sensitive to the ALK inhibitor crizotinib (Xalkori), but resistance ultimately occurs. In a phase I study reported in The New England Journal of Medicine, Alice T. Shaw, MD, PhD, of Dana-Farber/Harvard Cancer Center, Boston, and colleagues found that the more-potent ALK inhibitor ceritinib was capable of producing responses in advanced ALK-rearranged NSCLC, including in patients with prior crizotinib treatment and irrespective of the presence of ALK resistance mutations.1
Ceritinib is an oral ATP-competitive inhibitor of ALK tyrosine kinase that has been found to be 20 times more potent than crizotinib against ALK in enzymatic assays. Ceritinib also inhibits the IGF-1 receptor, against which it is manyfold less potent than against ALK, and unlike crizotinib it does not inhibit MET kinase activity. Ceritinib has shown marked antitumor activity against both crizotinib-sensitive and crizotinib-resistant tumors in xenograft models of ALK-rearranged NSCLC.
Study Details
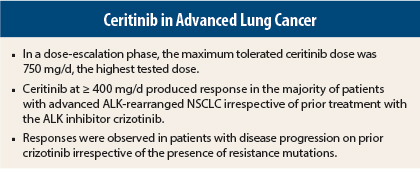
In the dose-escalation phase of the study, 59 patients with tumors harboring ALK alterations received ceritinib at doses of 50 to 750 mg once daily in 21-day cycles. In an expansion phase, 71 patients were treated at the maximum tolerated dose of 750 mg/d. Among the total of 130 patients, 122 had advanced NSCLC and had previously received cytotoxic chemotherapy (of the other 8 patients, 4 had breast cancer and 1 patient each had alveolar rhabdomyosarcoma, rectal adenocarcinoma, anaplastic large-cell lymphoma, and inflammatory myofibroblastic tumor).
In patients with NSCLC, the presence of ALK rearrangement was required in ≥ 15% of tumor cells as detected by fluorescence in situ hybridization (FISH) assay with the use of break-apart probes. Of the 122 patients with advanced NSCLC, 83 (68%) had received crizotinib.
In the dose-escalation phase, dose-limiting toxicities included diarrhea, vomiting, nausea, dehydration, elevated alanine transaminase (ALT), and hypophosphatemia. All dose-limiting toxicities resolved on discontinuation of treatment.
Response Rates
Among 114 patients with NSCLC who received ceritinib ≥ 400 mg/d, 66 (58%, 95% confidence interval [CI] = 48%–67%) had an objective response, including confirmed complete response in 1 (1%) and confirmed partial response in 65 (57%); 22% had stable disease, 11% had progressive disease, and response was not known in the 10% who withdrew early from the study.
Among the 78 patients receiving 750 mg daily, response rate was 59% (95% CI = 47%–70%). The response rate was 56% (95% CI = 45%–67%) among the 80 patients receiving ≥ 400 mg/d who had previously received crizotinib and 56% (95% CI = 41%–70%) among those receiving 750 mg/d. Among the 34 patients receiving ≥ 400 mg/d who had not received prior crizotinib, the response rate was 62% (95% CI = 44%–78%).
The investigators noted that “some responses were rapid and dramatic.” Responses were also observed in untreated central nervous system (CNS) lesions in patients who had received prior crizotinib treatment, with similar tumor responses observed in those who had not received crizotinib.
Response Duration and Survival
Among the 66 responding patients who had received ceritinib ≥ 400 mg/d, 64% had a response lasting ≥ 6 months. Median duration of response was 8.2 months; however, data for 47% of the patients with response were censored at the time of data cutoff.
Among 114 patients receiving ≥ 400 mg/d, median follow-up was 9.5 months and median progression-free survival was 7.0 months, with data for 38% of patients being censored. Median progression-free survival was 6.9 months in the 80 patients who had previously received crizotinib; in the 34 who had not received prior crizotinib, median progression-free survival was 10.4 months, with data censored for 53% and a median follow-up of 9.5 months. Progression-free survival was similar among the 64 patients with (6.9 months) and 50 patients without (7.0 months) CNS metastases at baseline.
Overall survival data were immature at the time of data cutoff, with data censored for 72% of patients. Overall survival at 12 months was 65%, and median overall survival had not been reached.
Response by Molecular Status
A total of 19 patients with NSCLC who had disease progression during crizotinib treatment had prestudy biopsies showing ALK rearrangement, including 12 with no additional alteration other than the original rearrangement, 5 with secondary resistance mutations in the tyrosine kinase domain, and 2 with ALK amplification. Tumor regression was observed in all patients regardless of molecular status, with confirmed response observed in 6 of the 7 patients with ALK gene amplification or secondary resistance mutation and in 7 of 12 patients with the original ALK alteration.
The investigators noted, “These findings suggest that the activity of ceritinib in patients whose tumors had progressed during crizotinib treatment may be independent of the underlying mechanism of acquired resistance.”
Toxicities
The most common adverse events of any grade were nausea (82%), diarrhea (75%), vomiting (65%), fatigue (47%), and increased ALT (35%). The most common grade 3 or 4 adverse events considered related to study drug were increased ALT (21%), increased aspartate transaminase (11%), diarrhea (7%), and increased lipase levels (7%), with all being reversible on discontinuation of treatment.
Four cases of interstitial lung disease were considered possibly related to ceritinib, with all resolving on discontinuation of ceritinib and use of standard treatments. One case of asymptomatic grade 3 prolongation of corrected QT interval was considered possibly related to ceritinib.
Dose reduction was required in 51% of patients (median duration of interruption = 7.3 days), and treatment was discontinued in 6% of patients due to adverse events. In patients receiving ceritinib at 750 mg/d, dose reduction was required in 62% of patients, with most reductions occurring in treatment cycle 3 or later. There were no treatment-related deaths.
The investigators concluded:
“Ceritinib was highly active in patients with advanced, ALK-rearranged NSCLC, including those who had had disease progression during crizotinib treatment, regardless of the presence of resistance mutations in ALK…. Among patients with ALK-rearranged NSCLC for whom crizotinib is no longer effective, more potent inhibition of the target by a structurally distinct ALK kinase inhibitor such as ceritinib can induce substantial and durable responses in the majority of cases. Confirmatory trials of the clinical activity of ceritinib in NSCLC are needed, involving patients who have received prior crizotinib treatment and those who have not.” ■
Disclosure: The study was funded by National Cancer Institute and V Foundation Translational Research grants, Be a Piece of the Solution, Evan Spirito Memorial Foundation, and by Novartis Pharmaceuticals. For full disclosures of the study authors, visit www.nejm.org.
Editors’ note: Ceritinib was recently approved by FDA for late-stage lung cancer (see page 62).
Reference
1. Shaw AT, Kim D-W, Mehra R, et al: Ceritinib in ALK-rearranged non–small-cell lung cancer. N Engl J Med 370:1189-1197, 2014.