Dermatologic Events in Oncology is guest edited by Mario E. Lacouture, MD, an Associate Member in the Division of Dermatology, Department of Medicine, at Memorial Sloan-Kettering Cancer Center, New York. He is a board-certified dermatologist with a special interest in dermatologic conditions that result from cancer treatments and the author of many publications, including the recently published Skin Care Guide for People Living With Cancer. The series is intended to offer oncologists guidance in recognizing and treating the skin toxicities associated with anticancer agents.
Epidermal growth factor receptor (EGFR) inhibitors block pathway activity in malignant cells as well as normal tissues, such as skin, hair, nails, and gut. They have been approved by regulatory agencies for the treatment of lung, pancreatic, colorectal, head and neck, medullary thyroid, and breast cancers.
These agents are either monoclonal antibodies (cetuximab [Erbitux], panitumumab [Vectibix], pertuzumab [Perjeta]) or small molecules (erlotinib [Tarceva], lapatinib [Tykerb], afatinib [investigational], vandetanib [Caprelsa]), and result in an acneiform rash (all grades) in 40% to 90% of patients. Whereas only 10% to 20% of patients develop grade 3 rash, decreased quality of life is seen across all grades of this untoward event.
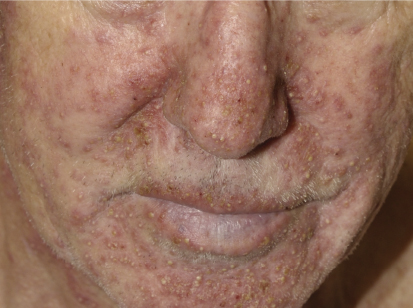
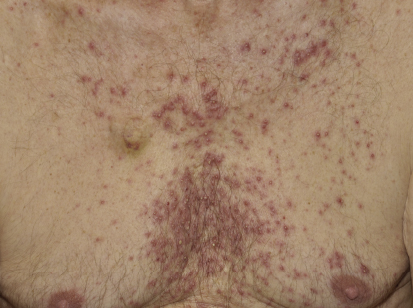
The acneiform rash is characterized by erythematous papules and pustules that develop on the face, scalp, and upper trunk within the first month in > 90% of patients. Not only does the rash have a cosmetic impact, but it is frequently associated with pain, infections, and pruritus.
The mechanism of rash development is primarily inflammatory, but approximately 30% of patients develop secondary bacterial infections, with hallmarks such as purulent discharge and yellow crusting. A correlation between presence and severity of rash and treatment response has been demonstrated, which underscores the need to treat patients with rash, as these are the individuals who could benefit the most from EGFR inhibitor therapy.
Treatment Recommendations
Prevention of acneiform rash caused by EGFR inhibitors includes topical corticosteroids (hydrocortisone 2.5%, alclometasone) and oral antibiotics (minocycline, doxycycline, or antibiotics covering skin flora) twice daily for at least the first 6 weeks. This results in a reduction of grade 2 and greater skin toxicities by more than 50%. In addition, these measures lessen the need to modify the dose of the EGFR inhibitor.
For treatment in patients who have already developed grade 1/2 rash, a similar approach, using topical corticosteroids (hydrocortisone 2.5%, alclometasone) and oral antibiotics (minocycline, doxycycline, or antibiotics covering skin flora) twice daily for at least 4 weeks is suggested. In cases where secondary infections are suspected, a bacterial swab culture should be performed in order to institute oral antibiotic therapy based on sensitivities.
For grade 3 rash, consider a dose interruption for 2 weeks or until the rash is grade ≤ 1. In addition, oral corticosteroids (methylprednisolone dose pack or prednisone 0.5 mg/kg for 7 days) may be beneficial. Also for grade 3 rash, topical corticosteroids and oral antibiotics should continue upon rechallenge with EGFR inhibitors. Application of a skin moisturizer and a broad-spectrum sunscreen with an SPF of at least 30 when going outside is recommended in all patients, since the rash is accompanied by dry skin and may be aggravated by sun exposure. ■
Disclosure: Dr. Lacouture is a consultant for AstraZeneca, Bayer/Onyx, Pfizer, GlaxoSmithKline, BMS, Roche, Genentech, and Amgen.