Treatment with the first-in-class PD-1/CTLA-4 bispecific antibody cadonilimab plus XELOX (capecitabine, oxaliplatin) chemotherapy demonstrated a survival advantage for patients with advanced gastric or gastroesophageal junction (GEJ) adenocarcinoma as first-line treatment, regardless of PD-L1 expression status, according to the results of the phase III COMPASSION-15 trial. The data were reported at the 2024 Annual Meeting of the American Association for Cancer Research (AACR) by lead author Jiafu Ji, MD, of Peking University Hospital in Beijing, China.1 The efficacy of cadonilimab in patients with low PD-L1 expression was notable.

Jiafu Ji, MD
In the intention-to-treat (ITT) population, the median overall survival with the combination therapy was 15 months vs 10.8 months in the chemotherapy-alone arm (P < .001). Median progression-free survival in the ITT population was 7 months for the combination therapy vs 5.3 months for chemotherapy alone (P < .001). Both overall survival and progression-free survival were significantly improved in patients with a PD-L1 combined positive score (CPS) of greater than or less than 5.
“Cadonilimab is the first PD-1/CTLA-4 bispecific antibody to demonstrate statistically significant and clinically meaningful overall survival and progression-free survival benefit in combination with chemotherapy vs chemotherapy alone in previously untreated patients with advanced gastric/GEJ cancers,” stated Dr. Ji.
“Cadonilimab may be a choice of treatment and a new standard of care for previously untreated patients with locally advanced or metastatic gastric/GEJ and patients with low PD-L1 expression,” Dr. Ji added. “The hazard ratios all favor cadonilimab. The survival advantage in patients with low PD-L1 expression has not been shown in other phase III trials with PD-L1 inhibitors such as nivolumab and pembrolizumab.”
Cadonilimab targets PD-1 and CTLA-4 simultaneously, two different approaches to jump-start the immune system. The bispecific antibody is designed to retain the efficacy benefit of the combination of PD-1 plus CTLA-4 with an improved safety profile over combination therapy. The drug is approved in China for second- and third-line treatment of recurrent and metastatic gastric or GEJ adenocarcinoma.
Dr. Ji explained that The National Comprehensive Cancer Network® Clinical Practice Guidelines in Oncology (NCCN Guidelines®) recommend anti–PD-1/PD-L1 antibodies such as nivolumab and pembrolizumab for those with high PD-L1 expression who have gastric or GEJ cancers. The data from COMPASSION-15 suggest that cadonilimab may have a role in treating patients with low PD-L1 expression.
Study Details
COMPASSION-15 was a double-blind trial that randomly assigned 610 patients to treatment with cadonilimab plus oxaliplatin and capecitabine or placebo plus the same chemotherapy. Chemotherapy with oxaliplatin/capecitabine was given every 3 weeks for up to six cycles. Cadonilimab at 10 mg/kg or placebo was given on day 1 of each cycle every 3 weeks.
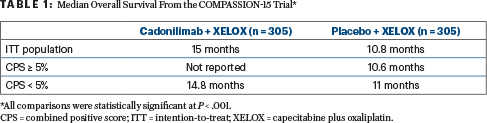
Participants with locally advanced unresectable or metastatic gastric/GEJ cancer were between the ages of 18 and 75, with a life expectancy of at least 3 months. Stratification factors included Eastern Cooperative Oncology Group performance status (0 vs 1), PD-L1 expression (CPS ≥ 5 or < 5), and presence or absence of liver metastasis. The primary endpoint was overall survival in the ITT population (Table 1).
Key Findings
The 18-month rate of overall survival in the ITT population was 45.8% vs 25.5% in cadonilimab and placebo recipients, respectively. In the CPS ≥ 5% population, the corresponding rate was 51.2% vs 23.5%, and in the CPS < 5% population, the corresponding rate was 44.1% vs 27.5%.
Progression-free survival benefits were observed with -cadonilimab/chemotherapy vs placebo/chemotherapy regardless of PD-L1 expression. In the ITT population, median progression-free survival was 7 months vs 5.3 months, respectively. In the CPS ≥ 5% population, the corresponding rate was 6.9 months vs 5.5 months, and in the CPS < 5% population, the corresponding rate was 6.9 months vs 4.6 months.
No new safety signals were observed in the trial. Grade 3 or higher treatment-related adverse events were reported in 65.9% of the combination therapy group vs 53.6% of the placebo group. Treatment-related adverse events leading to discontinuation of therapy occurred in 23.9% vs 6.6% of patients.
Cadonilimab is being evaluated in phase III trials of several tumor types, including as first-line treatment of cervical cancer and PD-L1–negative non–small cell lung cancer, as well as in adjuvant therapy for hepatocellular carcinoma.
Additional Commentary
Shivaani Kummar, MD, FACP, of the Knight Cancer Institute at Oregon Health & Science University, Portland, who moderated the press conference where the COMPASSION-15 data were presented,

Shivaani Kummar, MD, FACP
commented on the study: “It is interesting data, especially for patients with a PD-L1 expression level of [combined positive score] < 5. Data [for cadonilimab plus chemotherapy] would need to be confirmed in additional patient trials before this would be considered standard of care in the United States.”
DISCLOSURE: Dr. Ji reported no conflicts of interest. Dr. Kummar has served as a consultant for Bayer, Genome & Company, Genome Insight, Gilead Sciences, GI Innovation Inc, Harbour BioMed, Mundipharma, Oxford BioTherapeutics, Seagen, and SpringWorks Therapeutics; on a data and safety monitoring committee for Mirati Therapeutics; and holds stock in PathomIQ.
REFERENCE
1. Ji J, Shen L, Zi Z, et al: Cadonilimab plus chemotherapy versus chemotherapy as first-line treatment for unresectable locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma (COMPASSION-15): A randomized, double-blind, phase 3 trial. 2024 AACR Annual Meeting. Abstract CT006. Presented April 7, 2024.

