“Immunosignatures” may be well suited to enable the detection of ovarian cancer, researchers reported at the National Comprehensive Cancer Network (NCCN) 20th Annual Conference.1
“We developed a new concept for disease detection based on immunosignatures. From a drop of blood, HealthTell’s Immunosignature technology is capable of profiling the immune response to disease—in this case ovarian cancer. We are getting very high AUC [area under the receiver operating characteristic curve], with sensitivity, specificity, and overall accuracy all in the 90% range,” said Theodore M. Tarasow, PhD, Vice President of Product Development at HealthTell, the company developing the assay in San Ramon, California. The ASCO Post interviewed Dr. Tarasow at his poster presentation.
Natural Signal Amplification
The Immunosignature technology measures the body’s immune response to disease, taking advantage of the immune system’s natural signal amplification: more than 1,000 antibodies can be produced per B cell per second.
“The immune system amplifies the disease signal through B cells, which are creating thousands of antibodies per second that are specific to a given disease. This has advantages over looking for a protein or DNA biomarker that gets diluted once it goes out into the blood,” he explained.
The technology uses arrays of hundreds of thousands of unique peptides designed to broadly survey an individual’s antibody-binding repertoire from a drop of blood, serum, or plasma; it zeroes in on the signal generated by the disease response antibodies.
“The technology holds promise for detecting the presence of virtually any disease that generates a significant B-cell response,” Dr. Tarasow suggested. “For cancer, the technology has been demonstrated to distinguish patient samples representing several different cancers from each other, as well as from noncancer controls, with high accuracy.”
Highly Accurate in Ovarian Cancer Detection
To determine the feasibility of using the Immunosignature technology to detect ovarian cancer, the researchers tested samples from 39 patients with biopsy-confirmed ovarian cancer (stages IA to IV) and 49 age-matched controls with benign breast disease. They chose this control group because it may demonstrate inflammation, and the researchers wanted to be sure the immunosignature did not simply reflect an inflammatory process.
They identified peptides with statistically significant differences in binding signal between cases and controls and trained a support vector machine model as the classification algorithm using the 500 most significant features. Classification performance characteristics were evaluated using cross-validation techniques.
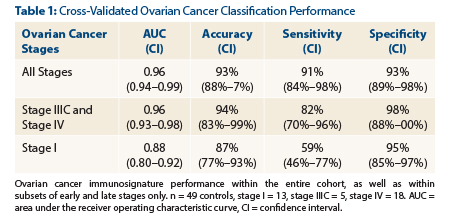
For all stages, the AUC was determined to be 0.96, sensitivity for detecting ovarian cancer was 91%, specificity was 93%, and overall accuracy was 93%. The cross-validated ovarian cancer classification performance, by stage, is shown in Table 1.
The next step is to evaluate the immunosignatures in larger, more diverse cohorts and to validate the findings in blinded studies, which are underway.
Dr. Tarasow said the test will probably be most useful in a high-risk population, not for general population screening, where incidence rates are very low. He noted that in future studies, they can power the cohorts enough to separate among stages and cell histologies. ■
Disclosure: Dr. Tarasow reported no potential conflicts of interest.
Reference
1. Tarasow T, Haddad M, Legutki JB, et al: Detection of stage I-IV ovarian cancer from a drop of serum. NCCN Annual Conference. Presented March 12, 2015.