“The value of experience is not in seeing much, but in seeing wisely.”
—Sir William Osler

Syed Ali Abutalib, MD

Stephen J. Forman, MD
To complement The ASCO Post’s continued comprehensive coverage of the 2021 American Society of Hematology (ASH) Annual Meeting & Exposition, here are several abstracts selected from the meeting proceedings focusing on allogeneic transplantation for hematologic neoplasms in adults. For full details of these study abstracts, visit ashpublications.org.
Acute Myeloid Leukemia
ABSTRACT 414: Retrospective analysis by the Center for International Blood and Marrow Transplant Research (CIBMTR): Prompt complete remission plus consolidation therapy yields improved survival after allogeneic hematopoietic cell transplantation (allo-HCT) for patients with acute myeloid leukemia (AML) receiving a myeloablative conditioning regimen and not a reduced-intensity conditioning regimen.1
Background: An important and unanswered question concerns the impact of the number of conventional AML chemotherapy induction cycles to achieve first complete remission on transplant outcomes. Similarly, the outcomes of myeloablative conditioning and reduced-intensity conditioning regimens for allo-HCT and their relationship with pretransplant consolidation cycles for patients in first complete remission are also unknown.
Methods: In this retrospective analysis, the investigators assessed the impact of the number of induction and consolidation cycles and disease status on the success of allo-HCT in 3,113 patients with AML from 2008 to 2019. They followed two groups of patients who received either myeloablative or reduced-intensity conditioning–based allo-HCT:
Patients in first complete remission: A total of 1,473 patients with a median age of 47 years received myeloablative conditioning, and 1,162 patients with a median age of 63 years received reduced-intensity conditioning.
Patients with primary induction failure: About 328 patients received myeloablative conditioning, and 150 patients, reduced-intensity conditioning.
Results: The investigators reported the following key findings:
Number of induction cycles prior to allo-HCT: Multivariate analysis demonstrated that complete remission after one cycle led to higher overall survival vs two cycles (hazard ratio [HR] = 1.32; 95% confidence interval [CI] = 1.11–1.56; P < .01) or three or more cycles (HR = 1.47; 95% CI = 1.16–1.87; P < .01). Overall survival after two cycles or at least three cycles was similar (HR = 1.2; 95% CI = 0.87–1.4; P = .38). Higher treatment-related mortality was observed in patients receiving two or at least three cycles vs one induction cycle (HR = 1.34; 95% CI = 1.05–1.72; P < .02; Table 1).
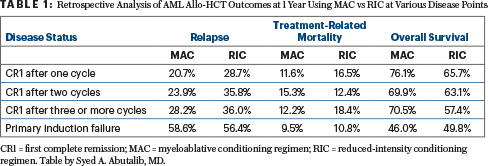
Consolidation therapy: Consolidation therapy after first complete remission was associated with improved overall survival vs no consolidation therapy (HR = 1.57; 95% CI = 1.24–1.99; P < .01). Nonetheless, the need for two or more induction cycles to achieve first complete remission plus consolidation therapy was associated with higher treatment-related mortality (HR = 1.34; 95% CI = 1.05–1.72; P < .02).
Primary induction failure: After myeloablative conditioning, overall survival and relapse were significantly worse in patients with primary induction failure compared with any patients in first complete remission (P < .01). Treatment-related mortality was similar for patients with primary induction failure vs those in first complete remission after myeloablative or reduced-intensity conditioning allo-HCT (Table 1).
Clinical Implications: This large retrospective analysis on behalf of the CIBMTR shows again that among patients eligible for allo-HCT, only one induction cycle to achieve first complete remission, particularly when combined with one consolidation therapy, is associated with better outcomes after myeloablative conditioning and not reduced-intensity conditioning. Naturally, more fit younger patients were able to receive more intensive conditioning regimens than less fit older adults. Compared with the poor outcomes of patients who received allo-HCT during refractory (active) disease, the outcomes of patients who received more than one induction cycle to achieve complete remission is somewhat better but remains unsatisfactory.
Future prospective studies are required to test different strategies that can improve the rates and quality (based on measurable residual disease [MRD] assessment) of remissions prior to and after allo-HCT using less toxic and more effective induction, conditioning, and maintenance therapies. Proper MRD assessment at different time points might be able to guide us on how best to approach patients with high-risk AML as well.
Myelofibrosis
ABSTRACT 169: Phase II study of ruxolitinib given pre–, peri– and post–allo-HCT for patients with primary or secondary myelofibrosis (ClinicalTrials.gov identifier NCT03427866).2
Background: In the pretransplant setting, ruxolitinib is often used to treat patients with symptomatic myelofibrosis who have splenomegaly for disease control and to facilitate hematopoietic engraftment. In most cases, the drug is initiated in the last 2 to 3 months before allo-HCT and is titrated to the maximum tolerated dose. A careful weaning process starts a few weeks prior to transplant conditioning to avoid a withdrawal/rebound phenomenon, with complete cessation of the drug a day prior to the conditioning regimen. There is growing interest in also using ruxolitinib peri– and post–allo-HCT given its efficacy in myelofibrosis and graft-vs-host disease. The use of ruxolitinib pre–, peri–, and post–allo-HCT may facilitate engraftment and allow better control of graft-vs-host disease and myelofibrosis.
Methods: This ongoing multicenter study utilizes ruxolitinib during and up to 12 months after reduced-intensity conditioning allogeneic transplant using fludarabine and melphalan with tacrolimus and methotrexate for graft-vs-host disease prophylaxis. The accrual goal is 48 patients with 1-year graft-vs-host disease–free and relapse-free survival as the primary endpoint. Secondary endpoints include overall and progression-free survival, engraftment, and incidence of acute and chronic graft-vs-host disease. The peripheral blood grafts in this current interim analysis of 26 patients were 7 of 8 (12%) or 8 of 8 (88%) human leukocyte antigen (HLA)-matched. At the time of allo-HCT, 58% had JAK2 mutations; 12%, CALR mutations; 12%, MPL mutations; and 35%, ASXL1 mutations.
Results: The preplanned interim analysis showed that with a median follow-up among survivors of 12 months (range = 3–24 months), 1-year graft-vs-host disease–free and relapse-free survival was 65%. The 1-year overall survival, progression-free-survival, and cumulative incidence of nonrelapse mortality and disease relapse were 77%, 71%, 13%, and 17%, respectively. There were no unexpected toxicities related to ruxolitinib therapy.
All but one patient achieved successful neutrophil engraftment, with a median time to neutrophil engraftment of 15 days (range = 11–38 days). Median day +30 donor all cell chimerism was 100% (range = 95%–100%). There was no grade IV acute graft-vs-host disease and one case of grade III acute graft-vs-host disease. The cumulative incidence of moderate to severe chronic graft-vs-host disease was 5%. All but one patient, who remains in remission at last follow-up, no longer had mutations detected by next-generation sequencing at day +100.
Clinical Implications: The interim analysis demonstrated the safety of ruxolitinib administration pre–, peri–, and post–allogeneic transplant with favorable engraftment rates and no unexpected toxicities in patients with advanced myelofibrosis using peripheral blood grafts. It would be of interest to follow the results of this, and another recently reported pilot study by Ali et al.3 The results of these studies should be interpreted in the context of graft type, conditioning regimen, immunosuppressive regimen and dose, schedule, and duration of ruxolitinib therapy.
Lymphomas
ABSTRACT 174: Retrospective analysis by the CIBMTR and the European Society for Blood and Marrow Transplantation (EBMT): Haploidentical vs HLA-matched unrelated donor (8/8 HLA-matched) transplants using posttransplant cyclophosphamide in lymphomas (n = 2,155).4
Background: Posttransplant cyclophosphamide is a standard graft-vs-host disease prophylactic approach for haploidentical hematopoietic cell transplantation (haplo-HCT). Retrospective studies in patients with lymphoma showed lower chronic graft-vs-host disease in haplo-HCT with posttransplant cyclophosphamide–based prophylaxis compared with matched unrelated donor transplants using calcineurin-based prophylaxis (with or without antithymocyte globulin). Recent retrospective studies showed that using matched unrelated donors was better than haploidentical donors when posttransplant cyclophosphamide and reduced-intensity conditioning are used in acute lymphocytic leukemia, AML, or myelodysplastic syndromes (MDS). The relative value of transplantation with matched unrelated vs haploidentical donors, when both groups receive posttransplant cyclophosphamide, a calcineurin inhibitor, and mycophenolate mofetil graft-vs-host disease prophylaxis, is not known in the setting of lymphomas.
Methods: The majority of matched unrelated donor–HCT recipients and haplo-HCT recipients were given reduced-intensity or nonmyeloablative conditioning using a peripheral blood graft (n = 1,379; 64%) and a three-drug graft-vs-host disease prophylaxis (posttransplant cyclophosphamide plus a calcineurin inhibitor plus mycophenolate mofetil; n = 1,805; 84%). Hodgkin lymphoma was the most common indication (n = 899; 42%), followed by diffuse large B-cell lymphoma (n = 525; 24%), T-cell lymphoma (n = 328; 15%), mantle cell lymphoma (n = 234; 11%), and follicular lymphoma (n = 169; 8%).
Most patients had chemosensitive disease at the time of allogeneic transplantation (n = 1,781; 83%). Median follow-up among survivors was longer with haplo-HCT than matched unrelated donor–HCT (24 and 17 months, respectively).
Results: In a multivariate analysis, all outcomes favored matched unrelated donor–HCT over haplo-HCT. Some of the results are summarized in Table 2.
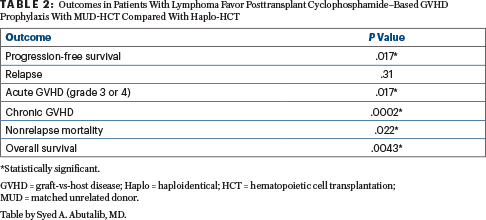
Clinical Implications: Retrospective data on behalf of the CIBMTR and EBMT demonstrate that patients with lymphoma receiving matched unrelated donor transplants with posttransplant cyclophosphamide have better outcomes than those with haploidentical transplants. These data extend and confirm the importance of donor-recipient HLA matching for allogeneic transplantation. We believe the matched unrelated donor approach remains the preferred second-donor choice in the absence of an HLA-matched sibling donor, even in patients with lymphoma. However, haplo-HCT with posttransplant cyclophosphamide–based graft-vs-host disease prophylaxis seems to be a valuable alternative for patients considered for allo-HCT who lack an HLA-matched donor.
Graft-vs-Host Disease Prophylaxis
ABSTRACT 101: Optimizing posttransplant cyclophosphamide dose and timing in the myeloablative conditioning setting: Phase I/II study of reduced dosing of posttransplant cyclophosphamide after HLA-haploidentical bone marrow transplantation (NCT03983850).5
Background: Posttransplant cyclophosphamide has been highly successful at preventing severe acute and chronic graft-vs-host disease after allo-HCT. However, the standard posttransplant cyclophosphamide dose (50 mg/kg/d) and timing (given on days +3 and +4) have been questioned. The hypothesis that standard clinical dosing may be higher than necessary and potentially comes at the expense of increased toxicity, delayed engraftment, and impaired immune reconstitution was tested by using reduced posttransplant cyclophosphamide dosing in the myeloablative conditioning setting in this trial.
Methods: All patients received myeloablative conditioning with fludarabine/busulfan, HLA-haploidentical marrow, and graft-vs-host disease prophylaxis with posttransplant cyclophosphamide (dose and timing based on dose level), mycophenolate mofetil (days +5 to +35), and sirolimus (days +5 to +80; instead of tacrolimus). The first five patients received posttransplant cyclophosphamide at 50 mg/kg/d on days +3 and +4 (standard dosing, dose level 1) for comparative data. This was followed by a 3+3 dose de-escalation design testing 25 mg/kg/d on days +3 and +4 (experimental, dose level 2) and 25 mg/kg on day +4 alone (experimental, dose level 3), followed by a phase II expansion cohort at the better experimental dose level. The primary endpoint and dose-limiting toxicity for the dose de-escalation was grade III to IV acute graft-vs-host disease.
Results: Phase I enrolled 19 patients, and the phase II expansion has enrolled 13 of 14 patients, 9 of whom have sufficient follow-up (60 days) to be considered evaluable for the primary endpoint of grade III to IV acute graft-vs-host disease. No grade III to IV acute graft-vs-host disease was seen at either experimental dose level (2 or 3). There have been no cases of grade II to IV acute graft-vs-host disease at dose level 2.
Based on more reliable early engraftment with less intense and shorter duration of engraftment fevers, dose level 2 (25 mg/kg/d on days +3 and +4) was selected for phase II. Engraftment of neutrophils and platelets was faster and most consistent with dose level 2. Primary graft failure was seen in one patient at dose level 2, and relapse prior to engraftment was seen in one patient at dose level 3. At dose level 2, there have been three cases to date of chronic graft-vs-host disease requiring systemic immunosuppression among the 13 engrafting patients with at least 100 days of follow-up.
Mucositis was less severe and shorter in duration for both experimental dose levels when compared with standard posttransplant cyclophosphamide dosing. Cytomegalovirus reactivation requiring preemptive therapy was less frequently seen after lower posttransplant cyclophosphamide dosing. Symptomatic BK virus–associated cystitis in at-risk patients was shorter in duration for patients receiving lower doses of posttransplant cyclophosphamide.
Clinical Implications: De-escalating posttransplant cyclophosphamide exposure is feasible in patients who receive myeloablative conditioning haplo-HCT. Two-day dosing of posttransplant cyclophosphamide at 25 mg/kg/d appears to allow for more consistent early engraftment and protection against protracted engraftment fevers compared with day +4 alone. However, these results are not conclusive and require longer follow-up with larger patient cohorts.
Of further interest is a recent publication from the Japan Study Group for Cell Therapy and Transplantation, which reported results of two consecutive prospective multicenter phase II studies to evaluate the safety and efficacy of posttransplant cyclophosphamide at 40 mg/kg/d on days 3 and 4, tacrolimus, and mycophenolate mofetil in 137 patients who underwent peripheral blood (not marrow) reduced-intensity consolidation haplo-HCT.6 The incidences of grade II to IV acute graft-vs-host disease, III to IV acute graft-vs-host disease, all-grade chronic graft-vs-host disease, and moderate to severe chronic graft-vs-host disease at 2 years were 26%, 5%, 35%, and 18% in the first study and 23%, 1%, 28%, and 15% in the second study, respectively. The rates of patients who were off immunosuppressants and survived without a relapse at 2 years were 83% and 76%.
Longer follow-up and comparative larger studies are needed to understand whether de-escalated posttransplant cyclophosphamide is equivalent or superior to the standard dosing schedule in different haplo-HCT settings.
Myelodysplastic Syndrome and Germline Mutations
ABSTRACT 320: Deleterious germline variants are present in patients with MDS of all ages treated with related allo-HCT.7
Background: Setting the diagnosis of myeloid neoplasms with germline predisposition is becoming increasingly recognized as crucially important for both the donor and recipient since it may (1) dictate the selection of donor for allo-HCT, (2) determine the conditioning regimen in selected cases, (3) enable relevant prophylactic measures and early intervention, and (4) facilitate genetic counseling and follow-up of at-risk family members.
Although the majority of MDS and AML cases are sporadic, the introduction of next-generation sequencing into the diagnostic workup has revealed that hereditary MDS and AML are more common than previously thought. Estimates suggest that about 5% to 15% of adults and 4% to 13% of pediatric patients with MDS or AML carry germline pathogenic variants in cancer susceptibility genes.8 In 2016, myeloid neoplasms with germline predisposition were included as a new dedicated entity in the revision of the World Health Organization classification of myeloid neoplasms.
Methods: The investigators performed whole-exome sequencing in 404 donor and MDS allo-HCT recipients (donor-recipient pairs) augmented with spike-in probes covering noncoding regions known to contain inherited risk alleles. Germline status was confirmed by the presence of a variant in the MDS patient and related donor and for variants previously only seen as germline alleles with a variant allele frequency of 40% to 60%.
Results: An analysis identified pathogenic germline variants in 7% of patients with MDS in all age deciles, from age 11 to 71, who were more likely to develop higher-grade (biologically more aggressive) MDS (43% vs 25%; P = .04). The 7% overall frequency of deleterious germline variants is likely an underestimate due to the following reasons:
- The study was constrained in calling germline variants as those detectable in both the donor (family) and recipient with MDS. (Only peripheral blood samples were available from the CIBMTR.)
- The relatives with germline variants that lead to cytopenias or other clinical features may have been excluded as transplant donors.
- Some aberrations deemed variants of “uncertain significance” are pathogenic but will require segregation or functional studies to upgrade them into deleterious categories.
- Given the small numbers, the transplant outcome measurements are underpowered.
- Deleterious variants in five bone marrow failure syndrome–related genes were found predominantly in younger people, whereas middle-aged and older patients with MDS most often had deleterious variants in DDX41 (n = 4) and other cancer predisposition genes (n = 16).
Clinical Implications: The precise magnitude of risk of each germline variant or combined variants on transplant outcomes is not entirely clear or defined.9,10 A recommendation to incorporate screening for germline variants into the standard evaluation of allogeneic transplant recipients with myeloid neoplasms and their related donors would require synthesis of scientific evidence, technical feasibility, cost-effectiveness, and ethical considerations.
Dr. Abutalib is Director, Hematology and BMT/Cellular Therapy Programs; Director, Clinical Apheresis Programs; Cancer Treatment Centers of America, Zion, Illinois; Associate Professor, Rosalind Franklin University of Medicine and Science; and Founder and Co-Editor of Advances in Cell and Gene Therapy. Dr. Forman is Director, Hematologic Malignancies Research Institute; Professor, Department of Hematology & Hematopoietic Cell Transplantation; Co-Leader, Hematologic Malignancies Program; Principal Investigator, Lymphoma SPORE; and Director, T Cell Therapeutics Research Laboratory at City of Hope, Duarte, California.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca. Dr. Forman has stock or stock options with Lixte Biotechnology and Mustang Bio; and has received research support from and is a paid consultant for Mustang Bio.
REFERENCES
1. Boyiadzis M, De Lima M, Zhang M, et al: Prompt CR plus consolidation therapy yields improved survival after allogeneic transplantation for AML patients receiving myeloablative, but not reduced-intensity conditioning. 2021 ASH Annual Meeting & Exposition. Abstract 414. Presented December 12, 2021.
2. Hobbs G, Kim H, Bottoms AJ, et al: A phase II study of ruxolitinib pre-, during- and post-hematopoietic cell transplantation for patients with primary or secondary myelofibrosis. 2021 ASH Annual Meeting & Exposition. Abstract 169. Presented December 11, 2021.
3. Ali H, Tsai NC, Synold T, et al: Peritransplant ruxolitinib administration is safe and effective in patients with myelofibrosis. Blood Adv 6:1444-1453, 2022.
4. Mussetti A, Kanate A, Wang T, et al: Haploidentical vs. matched unrelated donor transplants using post-transplant cyclophosphamide for lymphoma. 2021 ASH Annual Meeting & Exposition. Abstract 174. Presented December 11, 2021.
5. McAdams M, Hyder M, Dimitrova D, et al: Phase I/II study of reduced dosing of post-transplantation cyclophosphamide after HLA-haploidentical bone marrow transplantation. 2021 ASH Annual Meeting & Exposition. Abstract 101. Presented December 11, 2021.
6. Sugita J, Kamimura T, Ishikawa T, et al: Reduced dose of posttransplant cyclophosphamide in HLA-haploidentical peripheral blood stem cell transplantation. Bone Marrow Transplant 56:596-604, 2021.
7. Feurstein S, Trottier A, Estrada-Merly N, et al: Deleterious germline variants are present in patients with myelodysplastic syndrome of all ages treated with related allogeneic stem cell transplantation. 2021 ASH Annual Meeting & Exposition. Abstract 320. Presented December 11, 2021.
8. Baliakas P, Tesi B, Wartiovaara-Kautto U, et al: Nordic guidelines for germline predisposition to myeloid neoplasms in adults: Recommendations for genetic diagnosis, clinical management and follow-up. Hemasphere 3:e321, 2019.
9. DeZern AE, Gondek LP: Stem cell donors should be screened for CHIP. Blood Adv 4:784-788, 2020.
10. Gibson CJ, Lindsley RC: Stem cell donors should not be screened for clonal hematopoiesis. Blood Adv 4:789-792, 2020.

