Gastric cancer appears to have a new druggable target: fibroblast growth factor receptor 2b (FGFR2b). Targeting FGFR2b with bemarituzumab plus chemotherapy led to clinically meaningful and statistically significant improvements in progression-free survival, overall survival, and response rate in the randomized phase II FIGHT trial of previously untreated patients with advanced gastric or gastroesophageal junction cancer.1
“The FIGHT trial is the first study to evaluate targeting overexpression of FGFR2b and the only randomized data set of any FGFR inhibitor in any malignancy,” said Zev A. Wainberg, MD, Associate Professor of Medicine and Surgery at the University of California Los Angeles, Co-Director of the UCLA GI Oncology Program, and Director of the Early Phase Developmental Research Program at the Jonsson Comprehensive Cancer Center at UCLA, who presented the findings at the 2021 Gastrointestinal Cancers Symposium.

Zev A. Wainberg, MD
FGFR2b is a member of the FGFR family and is a splice isoform of FGFR2. FGFR2b has been shown to be overexpressed in many cancers. Kinase inhibitors blocking FGFR have been approved for patients with FGFR fusions or translocations in several malignancies.
Bemarituzumab is a first-in-class humanized IgG1 monoclonal antibody that selectively binds to FGFR2b, inhibits ligand binding, and mediates antibody-dependent cell-mediated cytotoxicity.
Study Details
The global, randomized, double-blind, placebo-controlled phase II FIGHT trial included patients with previously untreated unresectable locally advanced or metastatic gastric cancer that was not HER2-positive. All enrolled patients had tumors that overexpressed FGFR2b on a centrally performed immunohistochemistry (IHC) assay or FGFR2 gene amplifications by circulating tumor DNA (DNA). Of 910 patients who were prescreened globally, 30% of patients were considered FGFR2b overexpressed, which was based on IHC in about 95% of patients and amplified by circulating tumor DNA in about 16%. For about 13% of patients, these tests were both positive.
Patients were treated with modified FOLFOX6 (fluorouracil, leucovorin, oxaliplatin) and randomly assigned 1:1 to bemarituzumab at 15 mg/kg or placebo every 2 weeks with one additional bemarituzumab dose of 7.5 mg/kg on day 8. The primary endpoint was investigator-assessed progression-free survival; secondary endpoints were overall survival, response rate, and rate of adverse events. Statistical significance was tested sequentially for these endpoints, with a hazard ratio (HR) of up to 0.76 required for progression-free survival.
KEY POINTS
- Bemarituzumab targets FGFR2b, which is overexpressed in up to 30% of patients with gastric or gastroesophageal junction cancer.
- In the phase II FIGHT trial, patients receiving bemarituzumab plus mFOLFOX6 had significant improvements in progression-free survival (HR = 0.68) and overall survival (HR = 0.58).
- Patients with the highest expression of FGFR2b (IHC 2+/3+ ≥ 10%) had even greater benefit with bemarituzumab plus chemotherapy (HR = 0.44 and 0.41, respectively).
- Bemarituzumab is associated with some corneal toxicity.
From Phase III to Phase II
The study was originally launched as a randomized phase III trial, with overall survival as the primary endpoint. It was amended to a randomized phase II study for several reasons, according to Dr. Wainberg.
“Primarily, we wanted to become more comfortable with the biomarker. The unknown number of patients who would have FGFR2b-positive disease played a role. It became clear that the IHC percentage was greater than expected, and we wanted to get more granularity about this marker. So, we looked at the data early, and it’s obviously very compelling. We did preserve the placebo control and the centralized IHC and circulating tumor DNA component. The next step is to do the phase III trial as originally planned,” he explained.
Primary and Secondary Endpoints Met
Of 910 previously untreated patients with gastric or gastroesophageal junction cancer whose tumors were evaluated, 275 (30%) had FGFR2b-positive disease. Ultimately, 155 patients were randomly assigned, of whom 149 had FGFR2b-positive disease by IHC and 26, by circulating tumor DNA. As of the data cutoff, still on treatment were 42 patients on the bemarituzumab/mFOLFOX6 arm and 27 patients on the control arm.
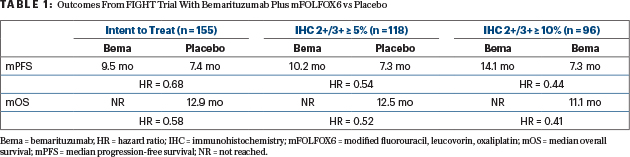
The primary endpoint was met, with an improvement in median progression-free survival from 7.4 months with placebo to 9.5 months with bemarituzumab (HR = 0.68; P = .07). The secondary endpoint of overall survival was also met, with the median not reached in the bemarituzumab arm compared with 12.9 months in the control arm (HR = 0.58; P = .03), which, according to Dr. Wainberg, is similar to that in historic controls. Patients with measurable disease saw an improvement in response rate from 40% to 53% with bemarituzumab; the median duration of response improved from 7.1 to 12.2 months with the targeted treatment, Dr. Wainberg reported.
“Of note, as FGFR2b expression increased, so too did benefit increase in groups receiving bemarituzumab. Hazard ratios continued to decrease,” he said (see Table 1). “That was true both for progression-free and overall survival.”
Safety Profile
Patients who received bemarituzumab experienced slightly higher grade 3 adverse events: 83% compared with 74% in the placebo arm. Overall, 34% of patients experienced toxicity requiring discontinuation of bemarituzumab, compared with 5% of patients on placebo, although exposure to bemarituzumab in both arms was similar (median of about 25 weeks). Grade 3 stomatitis increased from 1.3% in the placebo arm to 9.2 % with bemarituzumab.
“Of particular note was ocular toxicity, which is a known side effect of FGFR inhibitors,” he said. “The study required careful monitoring by ophthalmologists.”
Corneal events of any grade (mostly dry eye, keratitis, and punctate keratitis) were more common with bemarituzumab, 67% vs 10% of any grade and 24% vs 0% for grade ≥ 3. No cases of retinal detachment or hyperphosphatemia, however, occurred with the drug. Corneal toxicities resolved in 60% of patients at a median time to resolution of 27 weeks.
“In summary, we are very excited that this finding validates FGFR2 as an important target in gastric cancer. Our next step is to move forward with the phase III trial to confirm these important results,” Dr. Wainberg said. The results also support studies of bemarituzumab in other FGFR2b-positive tumor types, he added.
DISCLOSURE: Dr. Wainberg has served as a consultant or advisor to Array BioPharma, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Five Prime Therapeutics, Ipsen, Lilly, MacroGenics, Merck, Merck KGaA, Novartis, and QED Therapeutics; has received institutional research funding from Five Prime Therapeutics, Merck, Novartis, Pfizer, and Plexxikon; and has been reimbursed for travel, accommodations, or other expenses by Bayer, Lilly, and Merck.
REFERENCE
1. Wainberg ZA, Enzinger PC, Kang YK, et al: Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 in first-line treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). 2021 Gastrointestinal Cancers Symposium. Abstract 160. Presented January 15, 2021.

