Post hoc subanalyses of three KEYNOTE trials established the survival benefit of pembrolizumab in advanced gastric/gastroesophageal junction cancer with microsatellite instability–high (MSI-H) tumors or a programmed cell death ligand 1 (PD-L1) combined positive score (CPS) ≥ 10 (ie, the number of PD-L1–staining cells [tumor cells, lymphocytes, macrophages] divided by the total number of viable tumor cells, multiplied by 100). The KEYNOTE investigators presented the findings at the 2020 Gastrointestinal Cancers Symposium.
KEYNOTE-059, -061, and -062 Trials
The multicohort phase III KEYNOTE-059 trial included patients with at least two prior lines of therapy in its largest cohort and was a single-arm study. The phase III KEYNOTE-061 trial included patients with one prior line of treatment and compared pembrolizumab to paclitaxel. The phase III KEYNOTE-062 study evaluated pembrolizumab in newly diagnosed patients, either as a single agent or in combination with standard cisplatin and fluoropyrimidine chemotherapy compared to chemotherapy alone. In KEYNOTE-059 and KEYNOTE-061, patients were enrolled regardless of CPS status, but for KEYNOTE-062, patients were selected to have a CPS ≥ 1.

Joseph Chao, MD

Zev A. Wainberg, MD
An analysis presented by Joseph Chao, MD, of City of Hope, Duarte, California, examined pembrolizumab’s activity in patients with MSI-H advanced gastric/gastroesophageal cancer by prior line of therapy in these three trials.1 Zev A. Wainberg, MD, of the University of California, Los Angeles, presented outcomes in patients with a CPS ≥ 10.2
The total number of patients included in this analysis from all three trials was 1,357, of whom 67 had MSI-H status. The number of patients with a CPS ≥ 10 was 336. Median follow-up for these patients ranged from 5.6 months to 17.4 months at the time of analysis, depending on the trial.
Activity in MSI-H Patients
Clinically meaningful benefit was observed for progression-free and overall survival with pembrolizumab, Dr. Chao said (Table 1). “We see that, in the third line, pembrolizumab definitely leads to a long-term survival benefit. But even in the second line, and in newly diagnosed first-line MSI-H patients, median overall survival is also favored with pembrolizumab over chemotherapy,” Dr. Chao said.
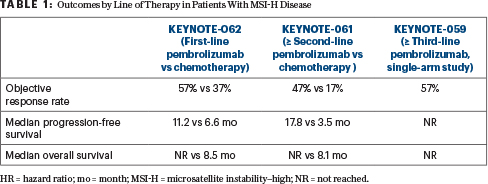
The current indication is for second line and beyond. A number of experts are now recommending that MSI testing be done upfront in patients with stage IV disease, rather than waiting to test in the second line, he said.
“I think that if we are going to consider a [programmed cell death protein 1 (PD-1)] inhibitor upfront, MSI-H status is the actionable biomarker,” Dr. Chao said, adding that, as a biomarker, “MSI-H seems to trump PD-L1.”
Patients With a CPS ≥ 1 and ≥ 10
The collective analysis of the use of pembrolizumab in the first, second, third, or greater line of therapy in patients with advanced gastric/gastroesophageal junction cancers across three KEYNOTE trials demonstrated improved overall survival compared with chemotherapy in patients with a CPS ≥ 10 status, said Dr. Wainberg. Interestingly, in the KEYNOTE-062 cohort of patients with a CPS ≥ 10, overall survival was improved despite lower response rates and shorter progression-free survival (Table 2).
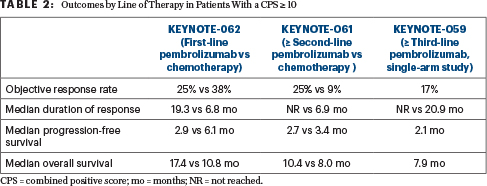
“The response rate with chemotherapy is higher in the front line, but in survival analyses (in this retrospective analysis) there is a much better median overall survival in the patients with a CPS ≥ 10 with pembrolizumab,” he said. It has become clear that a CPS ≥ 10 is a better biomarker than a CPS ≥ 1, but whether it is better than MSI-H may be a matter of debate. MSI-H status is observed in only 4% to 5% of patients, whereas about 20% of patients have a CPS ≥ 10. “It’s not as robust a biomarker as MSI-H, but it [selects for] a larger group of patients,” he said.
Dr. Wainberg further commented that, while pembrolizumab can be given to patients who have a CPS ≥ 1, he “feels a lot better” reserving the drug for patients with a CPS ≥ 10. With the new data, he is “more persuaded to use it earlier and with much less hesitation.” He cautioned that this is a retrospective post hoc analysis of patients with a CPS ≥ 10, but it is the most comprehensive efficacy analysis to date of CPS ≥ 10 in the population with gastric/gastroesophageal junction cancers.
DISCLOSURE: Dr. Chao has served in a consulting or advisory role for Amgen, AstraZeneca, Boston Biomedical, Daiichi Sankyo, Foundation Medicine, Lilly, MacroGenics, Merck, and Taiho Pharmaceutical; has participated in a speakers bureau for Merck; has received institutional research funding from Brooklyn Immunotherapeutics, Merck, and Novonco Therapeutics; and has been reimbursed for travel, accommodations, or other expenses by Foundation Medicine, MacroGenics, and Merck. Dr. Wainberg has served in a consulting or advisory role for Array BioPharma, AstraZeneca/MedImmune, Bayer, Bristol-Myers Squibb, Ibsen Pharmaceuticals, Five Prime Therapeutics, Genentech, Lilly, Merck, Merck KGaA, and Novartis; has received institutional research funding from Five Prime Therapeutics, Merck, Novartis, Pfizer, and Plexxikon; and has been reimbursed for travel, accommodations, or other expenses by Genentech, Lilly, Merck, Bayer, and Ibsen Pharmaceuticals.
REFERENCES
1. Chao J, Fuchs CS, Shitara K, et al: Pembrolizumab in microsatellite instability-high advanced gastric/gastroesophageal junction cancer by line of therapy. 2020 Gastrointestinal Cancers Symposium. Abstract 430. Presented January 23, 2020.
2. Wainberg ZA, Fuchs CS, Tabernero J, et al: Efficacy of pembrolizumab monotherapy vs chemotherapy for PD-L1–positive (CPS ≥ 10) advanced G/GEJ cancer in the phase II KEYNOTE-059 (cohort 1) and phase III KEYNOTE-061 and KEYNOTE-062 studies. 2020 Gastrointestinal Cancers Symposium. Abstract 427. Presented January 23, 2020.

