Targeted biologic agents have improved long-term outcomes in metastatic colorectal cancer, but debate continues as to their relative efficacy and proper sequencing. At the 2014 Gastrointestinal Cancers Symposium, a number of studies attempted to answer these questions by interrogating the clinical trial databases to assess not only efficacy, but “value.” A summary of their key findings is reported here.
Relative Merits of Targeted Agents
“Biologic therapies used in treating metastatic colorectal cancer are expensive, and debate continues about their ideal place in the therapeutic pathway. Individual trials inconsistently show their potential to improve outcomes. A meta-analysis was therefore performed to assess the impact in patients progressing after first-line therapy,” said David Chan, MD, of the Royal North Shore Hospital in St. Leonards, Australia.
Dr. Chan and colleagues evaluated the value of agents targeting the vascular endothelial growth factor (VEGF) receptor and epidermal growth factor receptor (EGFR) beyond the first-line setting by identifying trials comparing standard treatment to chemotherapy plus a biologic agent.1 They used several established databases as well as The Cochrane Library, proceedings of major oncology meetings, and unpublished data on trials of EGFR inhibitors (cetuximab [Erbitux], panitumumab [Vectibix]), VEGF receptor tyrosine kinase inhibitors (regorafenib [Stivarga]), anti-VEGF monoclonal antibodies (bevacizumab [Avastin]), and VEGF Trap (ziv-aflibercept [Zaltrap]).
The key results were as follows:
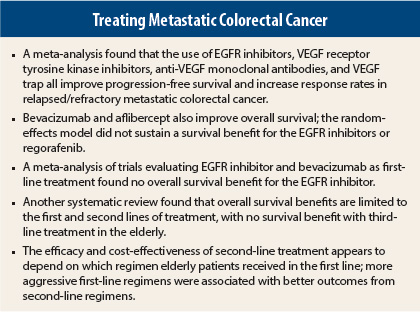
All of the targeted drug classes improve progression-free survival and increase response rates in relapsed/refractory metastatic colorectal cancer.
Bevacizumab and aflibercept are also associated with improved overall survival (as one class, hazard ratio [HR] = 0.79; P < .00001).
EGFR inhibitors as single agents or combined with chemotherapy, as well as regorafenib, have less certain effects on overall survival in random-effects modeling, likely due to crossover in some trials. Recent data regarding extended RAS testing may alter the results for cetuximab and panitumumab.
All classes increased grade 3/4 toxicity (more than twofold), but not grade 5 toxicity.
EGFR Inhibitors vs Bevacizumab
The FIRE-3 trial compared cetuximab and bevacizumab, showing improved overall survival, but not progression-free survival, with cetuximab.2 A systematic review of randomized controlled trials of first-line treatment, presented by Alexander Kumachev, MSc, BSc (Hons), a medical student at the University of Toronto, compared these agents and found that the results depended on the method of analysis.3 For their evaluation of 15 randomized trials involving 6,960 patients, the researchers used Bayesian pairwise and network meta-analyses to estimate the direct, indirect, and combined progression-free and overall survival hazard ratios comparing EGFR inhibitors to bevacizumab.
The direct pairwise analysis evaluated two trials—FIRE-3, which compared bevacizumab and cetuximab, and PEAK, which compared bevacizumab and panitumumab. In this analysis, the hazard ratio for progression-free survival was 1.00, while the hazard ratio for overall survival was 0.76 in favor of EGFR inhibitors.
The investigators combined this direct comparison with the indirect comparisons (13 trials of biologic vs chemotherapy alone) and the network meta-analysis (15 trials) and found the overall progression-free survival hazard ratio to be 1.10, a trend favoring bevacizumab. The hazard ratio for overall survival was 0.95, indicating no difference, ie, no overall survival benefit for the EGFR inhibitors.
“The results of direct pairwise meta-analysis, dominated by FIRE-3, suggested that EGFR inhibitors improved overall survival, without progression-free survival benefits, when compared to bevacizumab,” Mr. Kumachev noted. “However, the results from indirect or combined network meta-analyses, synthesizing all relevant data from the existing literature, did not confirm those findings,” he added.
“The findings of FIRE-3 may be due to chance or trial-specific reasons,” Mr. Kumachev suggested. “The results of the upcoming [Cancer and Leukemia Group B] (CALGB) 80405 trial will provide further direct evidence to help refine these estimates.”
Benefit Beyond Second Line?
Proceeding from first-line to second-line treatment with a chemotherapy regimen plus biologic significantly prolongs survival (HR = 0.659; P < .001) and colon cancer–specific survival (HR = 0.614; P < .001) in elderly patients, but treating beyond the second line does not. The findings are based on data from 5,129 metastatic colorectal cancer patients in the SEER-Medicare database, diagnosed between 2003 and 2007, prior to the advent of newer third-line agents.4
Investigators from the University of Maryland, Baltimore, conducted this large analysis and found that untreated patients had an adjusted median survival time of 6.8 months, but each line of chemotherapy/biologic led to longer survival: 11.9 months, 23.2 months, and 26.4 months for first-line treatment only, second-line treatment, and subsequent treatments, respectively. The respective colon cancer–specific mortality hazard ratios were 0.637, 0.398, and 0.364 (P < .001), in the study led by Nader N. Hanna, MD, of the University of Maryland team.
In an interview, senior author C. Daniel Mullins, PhD, questioned whether additional survival benefits of fewer than 3 months in elderly patients warrants the use of expensive new drugs. “Either the third-line treatment is not worth the cost, or we need new third-line regimens, because historically we are not deriving a survival benefit,” he said.
“Insurers will soon be passing along accountability not just to patients but to physician groups, and physicians will start asking, ‘Are we shifting survival enough to make third- and fourth-line regimens worth it?’” he continued.
Importance of First-Line Therapy
Dr. Mullins also led a study that evaluated the options recommended by the National Comprehensive Cancer Network, and found that “real-world survival benefits” of second-line treatments varied greatly, depending upon the first-line regimen, and so did cost-effectiveness.5
The study identified 3,211 patients at least 66 years old diagnosed with metastatic colorectal cancer between 2003 and 2009. Compared to patients who received first-line treatment, those who went on to receive second-line treatment lived longer, but the additional gain varied by the type of first-line regimen. Additional days associated with second-line treatment, according to their first-line therapy, were 172 for fluorouracil (5-FU)/leucovorin, 126 for oxaliplatin, 210 for irinotecan, and 220 for “others,” which included irinotecan/oxaliplatin or a biologic agent without oxaliplatin or irinotecan.
Cost also varied. Patients who received irinotecan/oxaliplatin or biologics without first receiving oxaliplatin or irinotecan gained higher survival benefits from further treatment, at a relatively lower cost. The highest incremental cost-effectiveness ratio of second-line treatment was for oxaliplatin in the first-line setting ($247,799), and the lowest was for “other” regimens ($92,422).
“Increasingly, the value proposition is something the health-care system is struggling with. Our paper broadens the discussion to show that it’s not as simple as asking whether we pay for second- or third-line treatment in the elderly patient, because the value of that second line is clinically interdependent on the first line,” Dr. Mullins explained.
“From the study, it appears that patients initially treated with more aggressive regimens (not just 5-FU/leucovorin) derive greater value from their second line of treatment than those treated less aggressively. While at this point, it’s hypothesis-generating only, this could challenge the way we historically treat the elderly, which is with a bias toward very simple regimens,” he suggested. n
Disclosure: Dr. Chan has received a travel grant from Amgen. Mr. Kumachev and Dr. Mullins reported no potential conflicts of interest. ■
References
1. Chan D, Segelov E, Shapiro J, et al: Meta-analysis of outcomes of VEGF and EGFR targeted biological therapy in relapsed metastatic colorectal cancer. 2014 Gastrointestinal Cancers Symposium. Abstract 534. Presented January 18, 2014.
2. Heinemann V, von Weikersthal LF, Decker T, et al: Randomized comparison of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment of KRAS-wildtype metastatic colorectal cancer: German AIO study KRK-0306 (FIRE-3). 2013 ASCO Annual Meeting. Abstract LBA3506. Presented June 1, 2013.
3. Kumachev A, Yan M, Berry SR, et al: A comparison of biologics in first-line advanced colorectal cancer: A Bayesian network meta-analysis of EGFR inhibitors and bevacizumab. 2014 Gastrointestinal Cancers Symposium. Abstract 543. Presented January 18, 2014.
4. Hanna N, Woods C, Zheng Z, et al: Survival benefit associated with the number of chemotherapy/biologic treatment lines in 5,129 metastatic colon cancer patients. 2014 Gastrointestinal Cancers Symposium. Abstract 559. Presented January 18, 2014.
5. Mullins CD, Woldemichael A, Zheng Z, et al: Does first-line treatment impact the cost-effectiveness of second-line treatment for elderly metastatic colon cancer patients? 2014 Gastrointestinal Cancers Symposium. Abstract 585. Presented January 18, 2014.