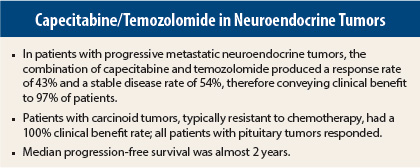
In an interim analysis of a phase II trial, 97% of patients with progressive metastatic neuroendocrine tumors achieved clinical benefit with the combination of capecitabine and temozolomide (CAPTEM). The results were reported at the 2014 Gastrointestinal Cancers Symposium by Robert L. Fine, MD, Director of the Experimental Therapeutics Program and Irving Association Professor of Medicine at Columbia University College of Physicians and Surgeons, Pancreas Center at Columbia, New York.1
“There is a great need for new treatments in well-differentiated neuroendocrine tumors, and there are a number of reasons why CAPTEM is an exciting combination,” Dr. Fine said in an interview with The ASCO Post.
“First, it’s an oral regimen of generic drugs. This will not cost a lot, yet it’s 5 to 10 times more efficacious than sunitinib [Sutent] or everolimus [Afinitor] and much less toxic. Second, it’s the first regimen to ever produce responses in all neuroendocrine subtypes, including carcinoid, a tumor that has a 0% to 5% response rate to chemotherapy.”
The ongoing median progression-free survival in this study—more than 22 months—is more than 150% greater than that reported with everolimus and sunitinib, he noted.
Dr. Fine and his team previously found that capecitabine and temozolomide are synergistic in inducing apoptosis in neuroendocrine tumor cell lines.
“We first reported in 2005 the use and mechanism of action of CAPTEM, and have since then seen promising retrospective results in patients who have failed all prior available therapies. This is our first prospective study.”
Small Study, Robust Result
The phase II study included patients with metastatic, well-to-moderately differentiated neuroendocrine tumors who had disease progression on long-acting octreotide (Sandostatin LAR), 60 mg, or who had negative octreotide scans, which is a negative prognostic factor. Patients received capecitabine at 1,500 mg/ m2 per day on days 1 to 14, and temozolomide at 150 to 200 mg/m2 per day on days 10 to 14 (capped at 2,500 mg per day), in a 28-day cycle.
The primary endpoint was response rate by RECIST criteria, with imaging performed after two cycles by two Columbia University radiologists with expertise in neuroendocrine cancer.
The study has accrued 38 patients with various neuroendocrine cancer subtypes, including carcinoid (typical and atypical), pituitary, pancreatic neuroendocrine, and medullary thyroid tumors. Of these patients, 28 of whom could be assessed for efficacy in the interim analysis. The overall response rate was 43%, including 11% complete responses, and 54% of patients achieved stable disease, resulting in a clinical benefit rate of 97%, Dr. Fine reported.
CAPTEM was very effective in tumors that are traditionally chemoresistant. For patients with carcinoid tumors (typical and atypical), the response rate was 42%, including 8% complete responses, and 58% of this group achieved stable disease. The median progression-free survival for this group exceeded 22 months.
“The most important thing is that CAPTEM works so well in carcinoid tumors, for which nothing else really works,” he said.
Response in Pituitary Tumors
The three patients with pituitary tumors all responded, and two (67%) had complete responses; their median progression-free survival exceeded 41 months. For all subtypes, ongoing median progression-free survival was 22 months, he reported.
The inclusion of pituitary tumors occurred as the result of Dr. Fine’s effort to treat a challenging patient who was referred to him. “I treat untreatable tumors, and I was called in to see this patient who had a tracheotomy and was ready to go on respiratory support. Her pituitary tumor was compressing the brain stem. She had had six surgeries, two radiation treatments, and chemotherapy.”
“I thought that since the pituitary gland secretes endocrine hormones and is derived from neural cells, we should try CAPTEM, and the tumor just melted away,” he noted. “She has had a complete response for 42 months, as has the second patient. The third pituitary tumor patient had a 75% reduction in tumor and died of other causes.”
Of the 28 assessable patients, 12 had died at the time of interim analysis, with a median overall survival of more than 29 months.
The most common grade 3/4 toxicities were lymphopenia (35%), hyperglycemia (65%, unlikely to be related), thrombocytopenia (3%), and diarrhea (3%). No hospitalizations, opportunistic infections, or deaths occurred from CAPTEM treatment. ■
Disclosure: Dr. Fine reported no potential conflicts of interest.
Reference
1. Fine RL, Gulati AP, Tsushima D, et al: Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. 2014 Gastrointestinal Cancers Symposium. Abstract 179. Presented January 17, 2014.