Two studies highlighted in press conferences and one presented during an invited lecture at the 2012 Gastrointestinal Cancers Symposium, held recently in San Francisco, suggest that early detection of pancreatic, esophageal, and colorectal cancers could soon improve.
 Enzyme Immunoassay Spots Pancreatic Cancer
Enzyme Immunoassay Spots Pancreatic Cancer
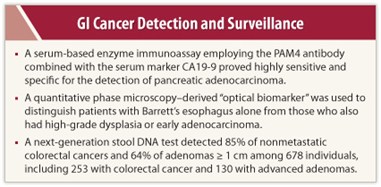
A serum-based enzyme immunoassay employing the PAM4 antibody combined with the serum marker CA19-9 proved highly sensitive and specific for the detection of pancreatic adenocarcinoma.1 The PAM4-based assay could be used for surveillance in patients at high risk for pancreatic adenocarcinoma, said David V. Gold, PhD, of the Garden State Cancer Center in Morris Plains, New Jersey.
The combined PAM4 antibody and CA19-9 assay was evaluated in more than 600 blood specimens, detecting 84% of pancreatic adenocarcinoma cases. This was significantly more sensitive than the single tests alone (P < .0001). Out of 50 cases of chronic pancreatitis, 9 (18%) were labeled positive by the assay, but this 18% rate of positivity does not necessarily represent false-positives, Dr. Gold suggested. The investigators believe that such cases may represent patients harboring occult pancreatic adenocarcinoma or who have precursor lesions producing the PAM4 biomarker.
‘Optical Biomarkers’ Detect High-risk Barrett’s Esophagus
In another study, investigators reported their use of an “optical biomarker” for surveillance of patients with established Barrett’s esophagus, aimed at distinguishing patients who have no dysplasia from those with high-grade dysplasia or early adenocarcinoma.2
The approach uses spatial-domain low-coherence quantitative phase microscopy. Using this technique, Randall E. Brand, MD, and colleagues at the University of Pittsburgh, Pennsylvania, identified at least three new features that are based on light reflectance and which represent abnormalities in cells. Using these biomarkers, the researchers constructed a prediction model and applied it retrospectively to archived tissue from 60 patients with Barrett’s esophagus who underwent biopsies. The test had an overall accuracy rate of 87%. It was 89% sensitive and 76% specific in distinguishing Barrett’s esophagus patients with high-grade dysplasia or adenocarcinoma from patients without dysplasia.
This approach could be used for monitoring Barrett’s esophagus patients to identify a subset at high risk who need more intensive surveillance or treatment. In addition, further studies are being performed to determine if this technology could identify a subset of patients in whom the surveillance interval can be lengthened.
Stool DNA Testing Becoming a Reality
 A third presentation, by David Ahlquist, MD, of the Mayo Clinic, Rochester, was an update on a next-generation stool DNA test (ColoGuard; Exact Science, Madison, Wisconsin) that might represent a promising approach for colorectal cancer.3 Stool DNA testing detects tumor-specific DNA alterations in cells that are continually shed into the stool from precancerous and cancerous lesions. In a large-scale, blinded case-control study of 678 individuals (pooled analysis of training and validation sets), including 253 persons with colorectal cancer and 130 with advanced adenomas, the investigators detected 87% of nonmetastatic colorectal cancers and 64% of adenomas > 1 cm, he reported.
A third presentation, by David Ahlquist, MD, of the Mayo Clinic, Rochester, was an update on a next-generation stool DNA test (ColoGuard; Exact Science, Madison, Wisconsin) that might represent a promising approach for colorectal cancer.3 Stool DNA testing detects tumor-specific DNA alterations in cells that are continually shed into the stool from precancerous and cancerous lesions. In a large-scale, blinded case-control study of 678 individuals (pooled analysis of training and validation sets), including 253 persons with colorectal cancer and 130 with advanced adenomas, the investigators detected 87% of nonmetastatic colorectal cancers and 64% of adenomas > 1 cm, he reported.
Neoplasms were detected in the proximal and distal colons with equal accuracy, and cancer stage did not affect detection rates. Importantly, sensitivity increased with adenoma size; the test detected 77% if larger than 2 cm and 91% if larger than 3 cm.
Dr. Ahlquist noted the test’s rational biologic basis, its “extraordinarily” high detection rates for curable cancers and precancers that are likely to progress, equal detection of lesions on both sides of the colon, and detection of flat polyps likely to be missed not only by fecal occult blood tests but also colonoscopy. It involves no diet or medication restrictions, no bowel preparation, and can be done at home using a stool sample. “It is user-friendly, affordable, and offers individuals unlimited access by mail,” he added. ■
Disclosure: Mayo Clinic has licensed intellectual property to and is a minor equity investor in Exact Sciences. Dr. Ahlquist is one of the inventors of the licensed technology and also serves as a scientific advisor to Exact Sciences. Dr. Gold and Dr. Brand reported no potential conflicts of interest.
References
1. Gold DV, Gaekcke J, Ghadimi BM, et al: Detection of early-stage pancreatic ductal adenocarcinoma (PDAC): Sensitivity, specificity, and discriminatory properties of the serum-based PAM4-immunoassay. 2012 Gastrointestinal Cancers Symposium. Abstract 151. Presented January 20, 2012.
2. Brand R, Rizvi S, Davison JM, et al: Use of optical biomarkers from nondysplastic metaplastic cells on the detection of high-grade dysplasia and adenocarcinoma from Barrett’s esophagus. 2012 Gastrointestinal Cancers Symposium. Abstract 14. Presented January 19, 2012.
3. Ahlquist DA: Stool DNA testing: A step closer to eradicating colorectal cancer. Invited presentation at the 2012 Gastrointestinal Cancers Symposium. Presented January 21, 2012.

