Updated results from the pivotal KEYNOTE-522 trial have confirmed the benefit of neoadjuvant therapy with pembrolizumab plus chemotherapy in patients with early triple-negative breast cancer.1 The results were presented at the 2021 San Antonio Breast Cancer Symposium by Peter Schmid, MD, PhD, Professor of Cancer Medicine at Barts Cancer Institute, Queen Mary University of London.

Patients [with nodal involvement] in the pembrolizumab group still had improved outcomes, suggesting that pembrolizumab provides benefit regardless of nodal status.— Peter Schmid, MD, PhD
Tweet this quote
“To evaluate the robustness of the study findings, we conducted five prespecified sensitivity analyses for event-free survival. These analyses showed a robust treatment benefit of neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab for previously untreated nonmetastatic triple-negative breast cancer. This benefit was generally consistent across a broad selection of prespecified patient subgroups, including those defined by nodal status and overall disease stage,” Dr. Schmid reported.
“The results further support pembrolizumab plus platinum-containing neoadjuvant chemotherapy, followed by adjuvant pembrolizumab after surgery, as a new standard-of-care treatment regimen for patients with high-risk early-stage triple-negative breast cancer,” he said.
KEYNOTE-522 Background
KEYNOTE-522 enrolled 1,174 patients with stage II or III -triple-negative breast cancer. Patients randomly assigned to the experimental arm (n = 784) received neoadjuvant treatment with pembrolizumab, paclitaxel, and carboplatin, followed by pembrolizumab, cyclophosphamide, and either doxorubicin or epirubicin, followed by surgery, and then adjuvant pembrolizumab. The control arm (n = 390) received neoadjuvant paclitaxel and carboplatin, followed by cyclophosphamide and either doxorubicin or epirubicin, then surgery.
The first analysis showed a significant 13.6% absolute increase in complete pathologic complete response with pembrolizumab/chemotherapy (P = .00055).2 The more recent fourth interim analysis showed a 37% reduction in events (P = .00031), and an absolute 7.7% improvement, based on a 3-year event-free survival rate of 84.5% compared with 76.8% with chemotherapy alone.3
New Sensitivity Analyses
The latest data are from five prespecified sensitivity analyses for event-free survival, including two that assessed the impact of different censoring rules and three that assessed the impact of different event definitions. These assessments included the use of alternate censoring rules,1 “new anticancer treatment for metastatic disease” as an event-free survival event,2 “positive margins at last surgery” removed from the event-free survival definition,3 “positive margins at last surgery” and “second primary malignancy” removed as an event-free survival definition, and “second breast malignancy” included as an event-free survival definition. For all these analyses, hazard ratios remained consistent with the primary analysis, ranging from 0.63 to 0.65, Dr. Schmid reported.
Treatment effects on event-free survival were also examined in
prespecified patient subgroups defined by nodal involvement (positive or negative), disease stage (II or III), menopausal status (premenopausal or postmenopausal), HER2 status (2+ by immunohistochemistry [IHC] but fluorescence in situ hybridization–negative, or 0–1+ by IHC), and lactate dehydrogenase (LDH; > upper limit of normal [ULN] or ≤ ULN).
KEY POINTS
- Five sensitivity analyses of KEYNOTE-522 confirm the multiple benefits previously observed for pembrolizumab plus chemotherapy in the neoadjuvant treatment of early triple-negative breast cancer.
- For all these analyses, hazard ratios remained consistent with the primary analysis, ranging from 0.63 to 0.65 for event-free survival.
- The benefit of pembrolizumab was seen regardless of nodal status, disease stage, and other factors.
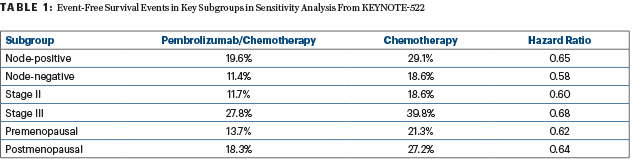
Event-free survival rates and hazard ratios for subgroups of most interest are shown in Table 1. Benefit in all subgroups of HER2 status and LDH levels had similar hazard ratios favoring the combination.
“As expected, patients with nodal involvement in both arms had poorer outcomes than those without involvement; nevertheless, patients in the pembrolizumab group still had improved outcomes, suggesting that pembrolizumab provides benefit regardless of nodal status,” Dr. Schmid commented. “Similarly, we see an event-free survival benefit with pembrolizumab irrespective of disease stage, which is in line with our previously presented subgroup analysis showing a treatment benefit irrespective of tumor size.”
DISCLOSURE Dr. Schmid reported financial relationships with Pfizer, Astellas, AstraZeneca, Novartis, OncoGenex, Roche, Merck, Boehringer Ingelheim, Bayer, Eisai, Puma, and Celgene.
REFERENCES
1. Schmid P, Cortes J, Dent R, et al: GS1-01 KEYNOTE-522 study of neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC: Event-free survival sensitivity and subgroup analyses. 2021 SABCS. Abstract GS1-01. Presented December 7, 2021.
2. Schmid P, Cortes J, Pusztai L, et al: Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 382:810-821, 2020.
3. Schmid P, Cortes J, Dent R, et al: KEYNOTE-522: Phase 3 study of neoadjuvant pembrolizumab plus chemotherapy versus placebo plus chemotherapy, followed by adjuvant pembrolizumab versus placebo for early-stage triple-negative breast cancer. ESMO Virtual Plenary. Abstract VP7-2021. Presented July 15, 2021.


