“There is progress whether ye are going forward or backward! The thing is to move.”
—Edgar Cayce
To complement The ASCO Post’s continued comprehensive coverage of the 2020 American Society of Hematology (ASH) Annual Meeting & Exposition, here are a few abstracts selected from the meeting proceedings focusing on novel therapies for chronic myeloid leukemia (CML). For full details of these study abstracts, visit ashpublications.org.
ASCEMBL Trial: Asciminib vs Bosutinib in Chronic-Phase CML
ABSTRACT LBA-4: Phase III ASCEMBL trial of asciminib vs bosutinib in previously treated chronic-phase CML (ClinicalTrials.gov identifier NCT03106779)1
Background: Asciminib targets both native and mutated BCR-ABL1, including the gatekeeper T315I mutation, via a novel allosteric (myristoyl site) mechanism. The study investigated whether asciminib could provide superior efficacy to bosutinib in chronic-phase CML beyond the second line, based on the clinical activity of asciminib monotherapy in heavily pretreated patients with CML in a phase I study.2

Syed Ali Abutalib, MD

Michael J. Mauro, MD
Methods: Adults with chronic-phase CML previously treated with at least two tyrosine kinase inhibitors were randomly assigned 2:1 to receive asciminib at 40 mg twice daily (n = 157) or bosutinib at 500 mg once daily (n = 76; Table 1). Randomization was stratified by major cytogenetic response (Philadelphia chromosome–positive metaphases ≤ 35%) status at baseline. Patients intolerant of their most recent tyrosine kinase inhibitor were eligible if they had BCR-ABL1 according to the International Scale developed for standardized reporting (BCR-ABL1 IS) > 0.1% at screening (19 patients with BCR-ABL1 IS < 1% enrolled). Patients with treatment failure on bosutinib were permitted to switch to asciminib. Patients with known bosutinib-resistant T315I or V299L mutations were excluded.
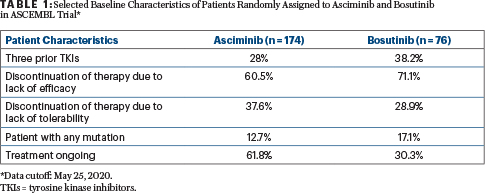
Results: The major molecular response (BCR-ABL1 IS < 0.1%) rate at 24 weeks was 25.5% with asciminib and 13.2% with bosutinib, meeting the primary endpoint. At 24 weeks, more patients on asciminib (17 and 14) than on bosutinib (4 and 1) achieved deep molecular response (MR4 and MR4.5, respectively).
Grade ≥ 3 adverse events occurred in 50.6% and 60.5% of patients receiving asciminib and bosutinib, respectively. The proportion of patients who discontinued treatment due to adverse events was lower with asciminib (5.8%) than bosutinib (21.1%).
Clinical Implications: In this first controlled study comparing treatments for patients with resistant/intolerant chronic-phase CML, asciminib demonstrated statistically significant and clinically meaningful superiority in efficacy compared with bosutinib at 24 weeks (primary endpoint), deeper molecular remission rates, and a favorable safety profile. It is important to note that fewer patients on asciminib discontinued their last tyrosine kinase inhibitor due to lack of efficacy, and fewer received at least three prior lines of tyrosine kinase inhibitor therapy compared with bosutinib. However, the most common cause of discontinuation of prior therapy was lack of efficacy. Multivariate analysis supported a similar benefit for asciminib over bosutinib based on major cytogenetic response status alone or with consideration of several other important covariates. Among the 24 patients who discontinued bosutinib due to lack of efficacy, 22 switched to asciminib. These results support the use of asciminib as a new treatment option in patients with CML, particularly in those resistant or intolerant to at least two prior tyrosine kinase inhibitors. (Also refer to Abstract 650.3)
JALSG CML212 Trial: Nilotinib vs Dasatinib in Chronic-Phase CML
ABSTRACT 45: Phase III JALSG CML212 trial of nilotinib vs dasatinib in newly diagnosed chronic-phase CML4
Background: Front-line therapy with second-generation tyrosine kinase inhibitors in patients with chronic-phase CML demonstrated higher efficacy as compared with imatinib, with fewer patients experiencing treatment failure and progression to advanced disease. However, limited information is available on selection of optimal second-generation tyrosine kinase inhibitors through direct comparison, including risk (adverse events) vs benefit (response, particularly molecular). This is a clinically important issue, since deep molecular response is a prerequisite to successfully discontinue a tyrosine kinase inhibitor.
Methods: The primary endpoint was the rate of cumulative achievement of MR4.5 by 18 months. Treatment doses were 300 mg twice daily for nilotinib and 100 mg daily for dasatinib. The BCR-ABL mRNA levels (IS scale reported) were monitored every 3 months. There were no significant differences in Sokal and European Treatment and Outcome Study (EUTOS) risk groups or frequencies of additional chromosomal abnormalities between the two arms.
Results:
In the intent-to-treat population, the cumulative achievement rates of MR4.5 by 18 months were 33.0% (75 of 227 patients; 95% confidence interval [CI] = 27.0%–39.6%) with nilotinib and 30.8% (70 of 227 patients; 95% CI = 24.9%–37.3%) with dasatinib, with no significant difference as assessed by the Cochran-Mantel-Haenszel test (P = .62). This finding was also confirmed in the per-protocol population (33.5% with nilotinib and 31.8% with dasatinib, P = .72).
The MR4.5 rates by 12, 24, and 36 months were 25.6%, 37.4%, and 40.5% with nilotinib and 23.4%, 36.6%, and 44.5% with dasatinib, respectively, with no significant difference. The progression-free survival, event-free survival, and overall survival did not differ between both arms regardless of Sokal and EUTOS risk groups.
“Symptomatic [COVID-19] infection was mild to moderate in the majority (~80%) of patients with CML.”— Syed Ali Abutalib, MD, and Michael J. Mauro, MD
Tweet this quote
In the safety population, grade 3 and 4 adverse events observed with at least 10% frequency were lipase elevation (11.5%) with nilotinib and neutropenia (12.8%) and thrombocytopenia (16.8%) with dasatinib. No significant differences were otherwise noted.
Clinical Implications: JALSG CML212 demonstrates that, in a direct randomized trial, nilotinib and dasatinib are equally effective for patients with a new diagnosis of chronic-phase CML in achieving MR4.5 as well as in achieving complete cytogenetic response and major molecular response in terms of both frequency and time to achievement with similar continuity. Risk/benefit, considering observed adverse events and response benefit, was comparable.
CANDID Trial: SARS CoV-2 Infection and CML
ABSTRACT 649: CANDID Study of SARS CoV-2 infection in patients with CML: Results from the International CML Foundation5
Background: Data specific to CML are essential to differentiate the impact of patient-, disease-, and therapy-specific factors on risk and outcomes as the COVID-19 pandemic continues. The primary goal of the CANDID study was to rapidly collect and analyze information on COVID-19 cases among patients with CML to define prognosis, risk factors, and outcome.
Methods: From March 12, 2020, the International CML Foundation sent an anonymous case collection form to its global network of physicians and partner organizations treating patients with CML. Forms were reviewed and cases tracked by its data manager. Updates were provided weekly. Only confirmed cases or those with a high level of suspicion were collected. Denominators regarding affected cohort (% tested, % affected in CML population) were not available in most instances.
At the time of presentation, 226 cases (increased from 110 cases in the abstract) of COVID-19 were reported to the International CML Foundation: 43% from Europe, 33%% from the Americas, 18% from Asia, 4.5% from Africa, and 0.5% from Oceania. A total of 201 cases were included in the analysis; among symptomatic (n = 188) cases, COVID-19 was diagnosed by polymerase chain reaction and/or serology in 166 patients (83%) and clinically suspected in 22 patients (11%).
Results: Investigators analyzed overall survival in the 170 patients with a known outcome according to gender, age, CML duration, type of CML treatment, line of tyrosine kinase inhibitor therapy, history of smoking, comorbidities, tyrosine kinase inhibitor interruption due to COVID-19, administration of any treatment against COVID-19 (eg, antimicrobial agents, steroids, oxygen, or anti–IL-6 antibodies), and economic status of country. Univariate analysis identified older age (≥ 65 vs < 65, 34% vs 13%, P = .00223) as a predictor for severe or critical COVID-19 infection. Gender, smoking history, treatment status (active or no tyrosine kinase inhibitor treatment), type of tyrosine kinase inhibitor, and,
notably, comorbidities were not significant risk factors for COVID-19 severity or death.
Clinical Implications: The CANDID study represents the largest global cohort study to date characterizing COVID-19 in patients with CML. Consistent with data published in larger unselected populations, advanced age predicts for more severe COVID-19 infection. With a larger data set analyzable since abstract publication, tyrosine kinase inhibitor treatment and generation (imatinib, second-generation agents) do not seem to be associated with infection severity or death at present. Symptomatic infection was mild to moderate in the majority (~80%) of patients with CML, and overall fatality from infection in cases with known outcomes was 10%. Additional cases and longer follow-up are needed to better ascertain the presence or absence of independent risk factors, the impact of COVID-19, and any potential impact of tyrosine kinase inhibitors in this population of interest.
DISCLOSURE: Dr. Abutalib has served on the advisory board for AstraZeneca. Dr. Mauro has received honoraria from, served as a consultant or advisor to, and been reimbursed for travel, accommodations, or other expenses by Bristol Myers Squibb, Novartis, Pfizer, and Takeda.
REFERENCES
1. Hochhaus A, Boquimpani C, Rea D, et al: Efficacy and safety results from ASCEMBL, a multicenter, open-label, phase 3 study of asciminib, a first-in-class STAMP inhibitor, vs bosutinib in patients with chronic myeloid leukemia in chronic phase previously treated with ≥ 2 tyrosine kinase inhibitors. 2020 ASH Annual Meeting & Exposition. Abstract LBA-4. Presented December 8, 2020.
3. Cortes JE, Hughes TP, Mauro MJ, et al: Asciminib, a first-in-class STAMP inhibitor, provides durable molecular response in patients with chronic myeloid leukemia harboring the T315I mutation: Primary efficacy and safety results from a phase 1 trial. 2020 ASH Annual Meeting & Exposition. Abstract 650. Presented December 7, 2020.
4. Matsumura I, Ohtake S, Atsuta Y, et al: Nilotinib vs. dasatinib in achieving MR4.5 for newly diagnosed chronic myeloid leukemia: Results of the prospective randomized phase 3 study, JALSG CML212. 2020 ASH Annual Meeting & Exposition. Abstract 45. Presented December 5, 2020.
5. Rea D, Mauro MJ, Cortes JE, et al: COVID-19 in patients with chronic myeloid leukemia (CML): Results from the International CML Foundation CML and COVID-19 (CANDID) study. 2020 ASH Annual Meeting & Exposition. Abstract 649. Presented December 7, 2020.
Dr. Abutalib is Associate Director of the Hematology and BMT/Cellular Therapy Programs and Director of the Clinical Apheresis Program at the Cancer Treatment Centers of America, Zion, Illinois; Associate Professor at Rosalind Franklin University of Medicine and Science; and Founder of Advances in Cell and Gene Therapy. Dr. Mauro is Leader of the Myeloproliferative Neoplasms Program, Attending Physician of the Leukemia Service, and Member of the Memorial Sloan Kettering Cancer Center and Professor of Medicine at Weill Cornell Medical College.

