CONFIRM Trial Reports Improvement in Survival With Nivolumab in Relapsed Malignant Mesothelioma

For the first time, a treatment has been shown to improve overall survival in heavily pretreated patients with relapsed malignant mesothelioma. In the phase III CONFIRM trial, single-agent nivolumab led to a significant improvement in both overall and progression-free survival, according to research presented at the International Association for the Study of Lung Cancer 2020 World Conference on Lung Cancer Singapore, which was held in a virtual format in January 2021.1
“CONFIRM met its co-primary endpoints of improved overall survival and progression-free survival with nivolumab vs placebo in relapsed malignant mesothelioma. Nivolumab monotherapy is a safe and effective treatment and should be considered a new treatment option,” said Dean A. Fennell, FRCP, PhD, Professor and Chair of Thoracic Medical Oncology, University of Leicester and University Hospitals of Leicester NHS Trust in the United Kingdom.

Dean A. Fennell, FRCP, PhD
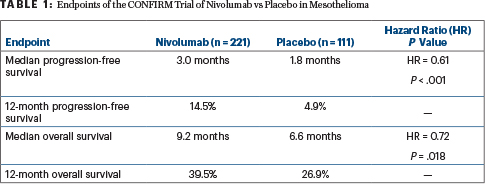
Overall survival was immature but was already significantly longer with nivolumab: median 9.2 months vs 6.6 months (hazard ratio [HR] = 0.72; P = .018). Investigator-assessed progression-free survival was also longer for nivolumab vs placebo: median 3.0 vs 1.8 months (HR = 0.61 P < .001), Dr. Fennell reported.
CONFIRM is the first ever placebo-controlled phase III trial of an agent targeting PD-1 in relapsed mesothelioma. According to Dr. Fennell, it is also the first trial to show an improvement in overall survival following the standard first-line doublet of pemetrexed and cisplatin or carboplatin.
“There are many patients who had first-line treatment, and getting access to a second-line therapy where there is evidence of efficacy is key,” Dr. Fennell told journalists in a press briefing. “This is an area of unmet need…. For patients with epithelioid mesothelioma, this could be an appropriate salvage strategy.”
CONFIRM Details
The investigator-led, placebo-controlled randomized phase III CONFIRM trial involved 24 centers in the United Kingdom. It enrolled 332 patients with previously treated, unresectable mesothelioma (pleural or peritoneal), randomly assigning them to receive nivolumab at 240 mg on day 1 of each 14-day cycle (n = 221) or placebo (n = 111). Participants were stratified by epithelioid vs nonepithelioid histology; 88% of both arms had epithelioid tumors, and 34% of patients had a tumor proportion score (TPS) of at least 1%, indicating expression of PD-L1. For more than half the patients, nivolumab was a third-line treatment.
The study used a hierarchic testing procedure, and its co-primary endpoints were investigator-assessed progression-free survival and overall survival. Progression-free survival data were mature after 310 events; overall survival data were immature, as 232 events had occurred out of a target of 291. “Some maturing of the survival data is required, but we have results from top-line data showing statistical significance,” Dr. Fennell commented.
Because of such positive findings, the data monitoring and ethics committee and trial steering committee recommended early release of the results in August 2020. Follow-up will continue until July 2021.
Study Endpoints Met
Significant benefits with nivolumab were observed in terms of both progression-free and overall survival (Table 1). Of note, PD-L1 expression did not appear to predict benefit among the 234 patients whose tumors were tested, and benefit was significant for epithelioid subtypes but not nonepithelioid subtypes, Dr. Fennell said.
For the epithelioid subtype, the median overall survival was 9.4 months with nivolumab vs 6.6 months with placebo, and the 12-month overall survival was 40.0% and 26.7%, respectively (HR = 0.71; P = .021). For the nonepithelioid subtype, these numbers were 5.9 months and 6.7 months, and 34.6% and 30.8%, respectively (HR = 0.79; P = .572). The lack of benefit in the nonepithelioid subtype might be due to the small number of patients (n = 39) in this group, he speculated.
For patients with PD-L1–positive disease (TPS ≥ 1%), the median overall survival was 8.0 months with nivolumab and 8.7 months with placebo (HR = 0.95; P = .864). For patients with PD-L1–negative disease, it was 9.0 months vs 6.4 months (HR = 0.74; P = .115). Dr. Fennell noted that whether PD-L1 positivity is predictive of benefit with nivolumab has been controversial in mesothelioma. “We’ve seen wide variation in its expression,” he noted. “At least in this setting and in this sample size, PD-L1 was neither predictive nor prognostic.”
Grade 3 or 4 adverse events were reported in 45% of the nivolumab arm and 42% of the placebo arm; serious adverse events were observed in 36% and 39%, respectively. Deaths related to a serious adverse event were recorded for 3.6% and 5.3%, respectively.
DISCLOSURE: Dr. Fennell reported no conflicts of interest.
REFERENCE
1. Fennell D, Ottensmeier C, Califano R, et al: Nivolumab versus placebo in relapsed malignant mesothelioma: The CONFIRM phase 3 trial. 2020 World Conference on Lung Cancer. Abstract PS02.11. Presented January 30, 2021.
Related Articles
Expert Point of View: Rina Hui, MBBS, PhD, and Melina Marmarelis, MD
“It has been a long time coming to see a positive randomized phase III study with a checkpoint inhibitor in relapsed mesothelioma,” said the study’s invited discussant, Rina Hui, MBBS, PhD, Clinical Professor of Medicine, Crown Princess Mary Cancer Centre, Westmead Hospital, University of Sydney,...
