
Shaji K. Kumar, MD
THE TREATMENT approaches for multiple myeloma, both newly diagnosed and relapsed disease, continue to undergo major transformation as new agents and combinations are being introduced.1 This change has been driven by the introduction of novel drug classes such as monoclonal antibodies, as well as newer agents from the existing classes of drugs such as proteasome inhibitors and immunomodulatory drugs. The monoclonal antibodies elotuzumab and daratumumab in particular have reshaped the treatment landscape of multiple myeloma during the past few years, given their efficacy in patients with multiple myeloma that has become refractory to the available drug classes.2-5
Challenge of Sequencing Therapies Over Time
GIVEN THE chronic nature of multiple myeloma today, patients continue to require repeated lines of therapy to maintain adequate disease control over long periods. As a result, the sequence of use of the different available therapies has taken on more significance. The uniform adoption of proteasome inhibitors and immunomodulatory drugs in the setting of newly diagnosed disease and the increasing use of continuous therapy in the initial treatment setting have a major impact on the therapeutic choices at the time of relapse.6 In particular, lenalidomide along with a proteasome inhibitor forms the backbone of initial treatment in older, frailer patients and is used for maintenance after autologous stem cell transplant in transplant-eligible patients, as well as the rest of patients after triplet induction.
As a result, increasingly, patients are refractory to lenalidomide at the time of initial relapse. Use of proteasome inhibitors in the first relapse, often in combination, also increases proteasome inhibitor–refractory disease in the later lines of therapy. Although several of the newer drugs, including the monoclonal antibodies have been studied in combination with lenalidomide in phase III studies, the clinical relevance of those studies remains limited given the pattern of upfront use of lenalidomide.2,3,7,8
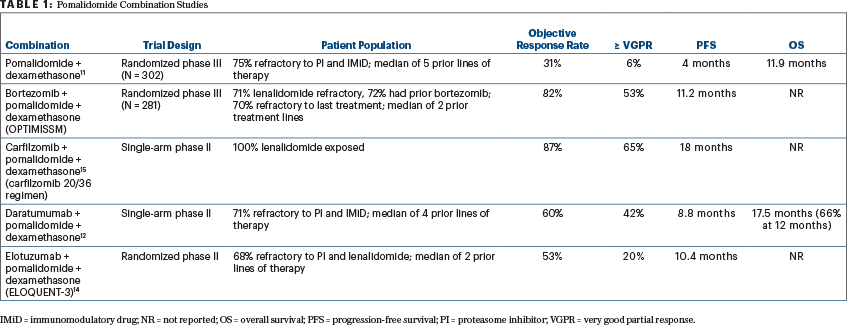
Pomalidomide is a next-generation immunomodulatory drug that has demonstrated efficacy in patients who are refractory to lenalidomide. Hence, this drug has been studied in combination with proteasome inhibitors and monoclonal antibodies in the relapsed setting.9-11 More recent randomized and single-arm trials have examined the combination of pomalidomide with proteasome inhibitors (bortezomib, carfilzomib, and ixazomib) as well as the monoclonal antibody daratumumab (Table 1).11-15
Key Findings From ELOQUENT-3
THE RANDOMIZED phase II ELOQUENT-3 trial, reported by Dimopoulos and colleagues and reviewed in this issue of The ASCO Post, has examined the efficacy of elotuzumab in combination with pomalidomide in patients with relapsed disease who are refractory to bortezomib and lenalidomide, a population that increasingly makes up the majority of patients with relapsed myeloma.14 The trial compared the three-drug combination of elotuzumab plus pomalidomide/dexamethasone (EPd) with pomalidomide/dexamethasone, demonstrating a higher response rate, deeper response, and improved progression-free survival, without a significant increase in toxicity. The overall response rate was 53% for EPd compared with 26% for pomalidomide/dexamethasone, including 20% vs 9% with a very good partial response or better. With 9.1 months of median follow-up, the median progression-free survival was significantly better with EPd at 10.3 months vs 4.7 months with pomalidomide/dexamethasone. The benefit was seen across the entire patient group regardless of age, prior lines of therapy, and disease risk status. Although overall survival was numerically better with EPd, the follow-up remains rather short.
The combination was well tolerated, with no significant increase in hematologic or nonhematologic toxicity with the addition of the antibody. The most common grade 3 or 4 adverse events were neutropenia, anemia, and hyperglycemia. Of note, the dosing strategy of elotuzumab differed in the current study compared with the lenalidomide combination, with use of a higher dose (20 mg/kg) every cycle after 2 cycles instead of 10 mg/kg every 2 weeks.
Comparing Trial Results With Caution
THE RESULTS of the current study need to be interpreted in the context of other pomalidomide combinations, especially with daratumumab or carfilzomib, when making the treatment choice in the clinic. Although the data with pomalidomide in combination with bortezomib from the OPTIMISSM trial appear promising, many patients at the time of relapse may be refractory or unable to tolerate bortezomib due to toxicities such as peripheral neuropathy. The results from the current trial and the trial with the daratumumab combination appear to be quite comparable, even though there are critical differences.12,14
The daratumumab study was a single-arm trial but had more heavily pretreated patients, with a median of 4 prior lines of therapy including one-third who had been exposed to carfilzomib. Although the response rate with daratumumab plus pomalidomide/dexamethasone was higher and deeper responses were seen, the overall progression-free survival was similar to that with EPd. In terms of tolerability, infusion reactions were seen less often with EPd, and hematologic toxicity appeared to be of a lesser magnitude as well. The once-every- 4-week dosing of elotuzumab also makes the administration logistics of the two regimens similar.
“Given the chronic nature of multiple myeloma today, patients continue to require repeated lines of therapy to maintain adequate disease control over long periods.”— Shaji K. Kumar, MD
Tweet this quote
Questions for the Future
WHAT THE TRIALS do not clearly convey is the activity of either of the monoclonal antibodies in combination with pomalidomide in a patient population that would have seen the other antibody in combination with lenalidomide. This will clearly be a question for the future, as daratumumab in combination with lenalidomide moves to the upfront setting in the treatment of newly diagnosed myeloma based on the recent data from the MAIA study. Depending on the results of the phase III ELOQUENT-1 study (which should be completed later this year), the converse may also be a relevant question for the future, if elotuzumab plus lenalidomide/dexamethasone becomes part of the initial therapy. For now, the promising results from the current trial clearly demonstrates an important role for the EPd combination in patients with myeloma that is refractory to bortezomib and lenalidomide. ■
Dr. Kumar is Professor of Medicine, Mayo Clinic, Rochester, Minnesota.
DISCLOSURE: Dr. Kumar has received research funding (with no personal payments) from Celgene, Takeda, Janssen, Bistol-Myers Squibb, Sanoffi, KITE, Merck, AbbVie, MedImmune, Novartis, Roche-Genentech, and Amgen; and is a consultant (with no personal payments) for Celgene, Takeda, Janssen, KITE, Merck, AbbVie, MedImmune, Genentech, and Amgen; and has received honoraria from Ono Pharmaceuticals, Reddys Laboratory, and Adaptive Technologies.
REFERENCES
1. Kumar SK, Rajkumar SV: The multiple myelomas: Current concepts in cytogenetic classification and therapy. Nat Rev Clin Oncol 15:409-421, 2018.
2. Lonial S, Dimopoulos M, Palumbo A, et al: Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med 373:621-631, 2015.
3. Dimopoulos MA, Oriol A, Nahi H, et al: Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375:1319-1331, 2016.
4. Lonial S, Weiss BM, Usmani SZ, et al: Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 387:1551-1560, 2016.
5. Palumbo A, Chanan-Khan A, Weisel K, et al: Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754-766, 2016.
6. Durie BG, Hoering A, Abidi MH, et al: Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 389:519-527, 2017.
7. Stewart AK, Rajkumar SV, Dimopoulos MA, et al: Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372:142-152, 2015.
8. Moreau P, Masszi T, Grzasko N, et al: Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 374:1621-1634, 2016.
9. Lacy MQ, Hayman SR, Gertz MA, et al: Pomalidomide (CC4047) plus low dose dexamethasone (Pom/dex) is active and well tolerated in lenalidomide refractory multiple myeloma. Leukemia 24:1934-1939, 2010.
10. Richardson PG, Siegel DS, Vij R, et al: Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: A randomized phase 2 study. Blood 123:1826-1832, 2014.
11. Dimopoulos MA, Palumbo A, Corradini P, et al: Safety and efficacy of pomalidomide plus low-dose dexamethasone in STRATUS (MM-010): A phase 3b study in refractory multiple myeloma. Blood 128:497-503, 2016.
12. Chari A, Suvannasankha A, Fay JW, et al: Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 130:974-981, 2017.
13. Shah JJ, Stadtmauer EA, Abonour R, et al: Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood 126:2284- 2290, 2015.
14. Dimopoulos MA, Dytfeld D, Grosicki S, et al: Elotuzumab plus pomalidomide and dexamethasone for multiple myeloma. N Engl J Med 379:1811- 1822, 2018.
15. Sonneveld P, Zweegman S, Cavo M, et al: Carfilzomib, pomalidomide and dexamethasone in patients with multiple myeloma refractory to bortezomib and lenalidomide: The EMN011 trial. Blood 132:801, 2018.


