Recent phase III findings support the antibody-drug conjugate belantamab mafodotin-blmf as a treatment option for patients with multiple myeloma in early relapse. Belantamab mafodotin is an antibody-drug conjugate targeting B-cell maturation antigen (BCMA) expressed on multiple myeloma cells, conjugated to the synthetic tubulin inhibitor monomethyl auristatin F as its toxic payload.
The addition of belantamab mafodotin to pomalidomide and dexamethasone (BPd) significantly reduced the risk of disease progression by 48% (P < .001), as compared to pomalidomide, bortezomib, and dexamethasone (PVd), in the phase III DREAMM-8 trial.1 The results accompany positive results of the phase III DREAMM-7 trial, which showed a strong benefit for adding belantamab mafodotin to bortezomib and dexamethasone (BVd), as compared to daratumumab, bortezomib, and dexamethasone after first relapse (hazard ratio [HR] = 0.41; P = .00049). An update presented at the 2024 ASCO Annual Meeting found the progression-free survival benefit extended to poor-prognosis subgroups, including those with disease refractory to lenalidomide and those with high-risk cytogenetic abnormalities.2
Patients in DREAMM-8 and DREAMM-7 were treated after just one or more lines of therapy, with DREAMM-8 requiring lenaliomide exposure, as compared to earlier studies in later lines. Both studies were published during the meeting in The New England Journal of Medicine.3,4
Multiple Positive Outcomes

Suzanne Trudel, MSc, MD
“Taken together with the results of phase III DREAMM-7, these results suggest that belantamab mafodotin combinations can be new standards of care at first relapse or later due to their robust efficacy, manageable safety, and ease of administration,” said Suzanne Trudel, MSc, MD, Professor, Department of Medical Oncology/Hematology, Princess Margaret Cancer Centre in Toronto.
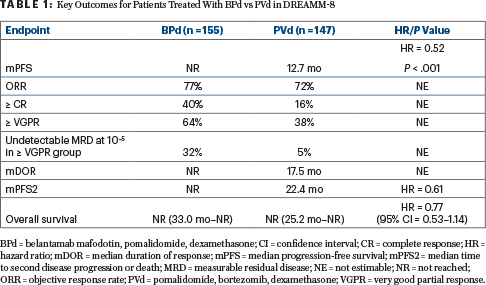
Median progression-free survival was not reached with BPd, but it was 12.7 months with PVd (HR = 0.52; P < .001). This translated into a 12-month progression-free survival rate of 71% vs 51%, respectively. The benefit was observed across challenging subgroups, Dr. Trudel noted, including patients with cytogenetic or functional high-risk disease (HR = 0.57), lenalidomide-refractory disease (HR = 0.45), and previous exposure to anti-CD38 therapy (HR = 0.49).
Multiple outcomes were improved with the belantamab mafodotin–containing triplet (Table 1). In addition to the strong progression-free survival benefit, BPd was associated with an early trend in overall survival benefit and greater depth of response, including undetectable measurable residual disease and durability.
ASCO Expert Oreofe O. Odejide, MD, MPH, Assistant Professor of Medicine, Dana-Farber Cancer Institute, Boston, noted at a press briefing that since today’s patients are exposed to many agents, resistance emerges, and they need novel therapies. “Based on these findings, belantamab is meeting the need for novel treatment…. The combination of belantamab, pomalidomide, and dexamethasone is poised to be a potential new treatment option for patients with relapsed/refractory myeloma,” Dr. Odejide commented.
History of Belantamab Mafodotin
In 2020, belantamab mafodotin received accelerated approval from the U.S. Food and Drug Administration after four or more prior lines of therapy, based on the results of DREAMM-2.5 However, DREAMM-3 failed to demonstrate a statistically significant progression-free survival benefit of belantamab mafodotin over the pomalidomide/dexamethasone doublet, and the drug was subsequently withdrawn from the market.6
The current phase III international trial evaluated belantamab mafodotin in 302 patients previously treated with at least one prior line of therapy, including lenalidomide but no BCMA-targeting agent or pomalidomide. The primary endpoint was progression-free survival per central independent review.

Oreofe O. Odejide, MD, MPH
Focus on Ocular Toxicities
In DREAMM-8, grade 3 or 4 adverse events occurred in 91% of the BPd arm and 73% of the PVd arm, “but exposure-adjusted rates were similar or lower in the BPd arm than in the PVd arm,” Dr. Trudel reported. There were 17 deaths in the BPd group and 16 in the PVd group. Global health status and quality of life remained stable in both arms.
Ocular events with BPd—the main concern with this drug—were primarily blurred vision and dry eye. These events were grade ≥ 3 in 43% and 17%, respectively, of the BPd cohort and 2% and 0%, respectively, in the control arm. Ocular events leading to dose interruptions or delays occurred in 83%, and 59% required dose reductions primarily in the form of increasing dosing interval, but only 9% discontinued the treatment because of ocular concerns.
A decrease in best-corrected visual acuity to 20/50 or worse was observed in 34% of BPd-treated patients, and 1% had a decrease to 20/200 or worse. Within these worsening subsets, 84% and 50%, respectively, resolved to normal after a first event, and 92% and 100%, respectively, improved. “Visual acuity changes that could affect activities of daily living were reversible in most patients,” she said. “And it’s interesting that 30% of patients in the PVd arm also reported ocular symptoms.”
Expert Point of View

Sagar Lonial, MD
Commenting on DREAMM-8, invited discussant Sagar Lonial, MD, Professor and Chair of Hematology and Medical Oncology at Emory University’s Winship Cancer Institute in Atlanta, said: “What we’ve seen here is a very interesting and exciting phase III trial…. It’s the longest progression-free survival we’ve seen with a pomalidomide/dexamethasone combination.”
The benefit may actually exceed that seen in DREAMM-7—where a median progression-free survival of almost 37 months was achieved with belantamab mafodotin-blmf, bortezomib, and dexamethasone—because of the interaction between the antibody-drug conjugate and the immunomodulatory drug, pomalidomide. This combination may have enhanced immune effects, he suggested.
Thoughts on Safety Profile
Ocular toxicity remains a concern. These adverse events are related to the drug’s monomethyl auristatin F payload, which causes the formation of cysts that can lead to (a largely reversible) loss of visual acuity. In the DREAMM series of trials, these side effects are managed with dose delays and/or reductions; however, it may be possible to reduce toxicity and retain the efficacy of belantamab mafodotin, Dr. Lonial maintained. In DREAMM-8, the belantamab mafodotin dose was 2.5 mg/kg for the first cycle and 1.9 mg/kg thereafter.
“Patients and physicians worry that missed doses may impact progression-free survival. We now have evidence that this is not true. Missed doses may actually result in better safety profiles and maintain the efficacy of the treatment,” he said. “This is a unique target and payload, and the efficacy may be superior with less toxicity…. I would argue that the current dosing schedule of this highly effective regimen is too much. We need to use less, less frequently, and do it in a way that preserves patient function.”
DISCLOSURE: Dr. Trudel has received honoraria from Amgen, BMS, GlaxoSmithKline, Janssen Oncology, Pfizer, Roche, and Sanofi; has had a consulting or advisory role with BMS, GlaxoSmithKline, and Roche; and has received institutional research funding from Amgen, BMS, Genentech, GlaxoSmithKline, Janssen, Pfizer, and Roche. Dr. Odejide reported no conflicts of interest. Dr. Lonial reported financial relationships with TG Therapeutics, Celgene, Bristol Myers Squibb, Janssen Oncology, Novartis, GlaxoSmithKline, Amgen, AbbVie, Takeda, Merck, Pfizer, Regeneron, and Sanofi.
REFERENCES
1. Trudel S, Beksac M, Pour L, et al: Results from the randomized phase 3 DREAMM-8 study of belantamab mafodotin plus pomalidomide and dexamethasone (BPd) vs pomalidomide plus bortezomib and dexamethasone (PVd) in relapsed/refractory multiple myeloma. 2024 ASCO Annual Meeting. Abstract LBA105. Presented June 2, 2024.
2. Mateos MV, Roak P, Hus M, et al: DREAMM-7 update: Subgroup analyses from a phase 3 trial of belantamab mafodotin + bortezomib and dexamethasone vs daratumumab, bortezomib, and dexamethasone in relapsed/refractory multiple myeloma. 2024 ASCO Annual Meeting. Abstract 7503. Presented June 3, 2024.
3. Dimopoulos MA, Beksac M, Pour L, et al: Belantamab mafodotin, pomalidomide, and dexamethasone in multiple myeloma. N Engl J Med. June 2, 2024 (early release online).
4. Hungria V, Robak P, Hus M, et al: Belantamab mafodotin, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. June 1, 2024 (early release online).
5. Lonial S, Lee HC, Badros A, et al: Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol 21:207-221, 2020.
6. Dimopoulos, MA, Hungria VTM, Radinoff A, et al: Efficacy and safety of single-agent belantamab mafodotin versus pomalidomide plus low-dose dexamethasone in patients with relapsed or refractory multiple myeloma (DREAMM-3): A phase 3, open-label, randomised study. Lancet Haematol 10:E801-812, 2023.

