In March 2022, Kristeleit et al reported the results of the ARIEL4 trial1 of rucaparib in relapsed BRCA-mutant ovarian cancer in The Lancet Oncology (summarized in this issue of The ASCO Post) and are to be congratulated on this accomplishment. This report, along with the almost simultaneous release of NRG-GY004 results by Liu et al in the Journal of Clinical Oncology,2 add to the information already released from SOLO-3 by Penson et al3 in describing expectations for clinical benefit when poly (ADP-ribose) polymerase (PARP) inhibitors are used instead of cytotoxic chemotherapy in this setting.

“Outside of the front-line setting, PARP inhibitor use is continued indefinitely, and this may negatively impact therapy after disease progression.”— Kathleen Moore, MD, MS
Tweet this quote
Although similar, these three trials do have notable differ-ences. SOLO-3 randomly assigned patients with recurrent platinum-sensitive ovarian cancer and at least two prior lines of platinum therapy to olaparib as compared with investigator’s choice of nonplatinum cytotoxic therapy.3 NRG-GY004 enrolled all patients with high-grade serous or endometrioid platinum-sensitive ovarian cancer after only one prior platinum treatment,2 and ARIEL4 allowed the inclusion of patients with platinum-resistant recurrences.1
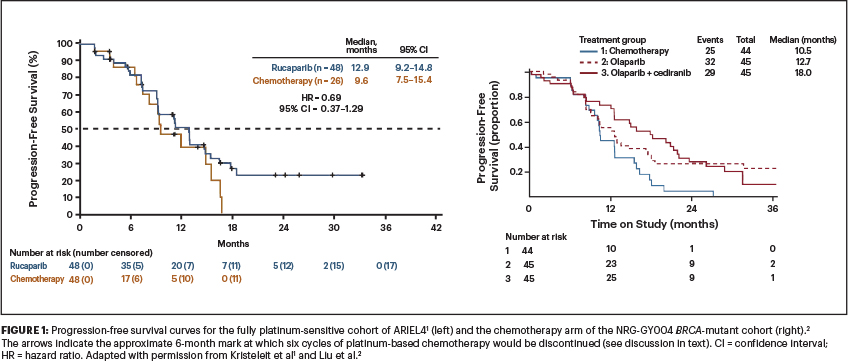
Table 1 lists these three trials (platinum-sensitive cohorts) and their outcomes and shows how consistently PARP inhibitors perform across the trials, with a median progression-free survival of approximately 13 months, as compared with platinum-based chemotherapy (in NRG-GY004 and the fully platinum-sensitive cohort of ARIEL4) at approximately 10 months.1-3
Caveats and Questions
Based on these trials, it is reasonable to say that PARP inhibitors may be considered an option in the platinum-sensitive ovarian cancer setting instead of chemotherapy in appropriately counseled patients. The big caveat to this is the fact that these studies were all done during a time when the standard of care for platinum-sensitive ovarian cancer changed to allow switch-maintenance PARP inhibitor therapy following induction platinum-based chemotherapy. Indeed, in NRG-GY004, a full 23% of patients randomly assigned to the chemotherapy arm received “nonprotocol” therapy, which primarily was standard-of-care PARP inhibitor maintenance.2
Question 1, therefore, is whether a PARP inhibitor is equivalent or superior to platinum-based chemotherapy when that platinum-based chemotherapy is followed by a PARP inhibitor. Figure 1 shows the progression-free survival curves for the fully platinum-sensitive cohort of ARIEL41 and the chemotherapy arm of the NRG GY004 BRCA-mutated cohort.2 The arrows indicate the approximate 6-month mark at which six cycles of platinum-based chemotherapy would be discontinued. How different would the curves look if PARP inhibitor maintenance had been initiated at that point instead? Can we say that PARP inhibitor treatment is equivalent to platinum-based chemotherapy in this setting, when it wasn’t tested vs modern treatment regimens? I would argue ‘no.’
The second question concerns how relevant the ARIEL4 study is now, given that the standard of care has so drastically changed—first with the movement of PARP inhibitors into the platinum-sensitive ovarian cancer maintenance space and more recently with PARP inhibitor maintenance therapy moving into the front-line space?4-6 Of course, this study is relevant from a rucaparib development standpoint, and the results of the ATHENA monotherapy trial presented at the 2022 ASCO Annual Meeting will likely move rucaparib into greater use than in current practice. However, demonstrating efficacy in late-line therapy is stepping back to the first approvals of PARP inhibitors based on Study 1 (olaparib),7 ARIEL2 (rucaparib),8 and QUADRA (niraparib).9 Although PARP inhibitors work in this setting, the greatest magnitude of benefit is earlier and—at least for patients with BRCA-associated ovarian cancer—should be given in the front line.
“[The study by Kristeleit et al] is important in that it … points to pretreatment evaluation for reversion mutations as ‘a must do’ if using PARP inhibitors in the recurrent setting.”— Kathleen Moore, MD, MS
Tweet this quote
Acknowledging that overall survival data in this setting have not yet been reported, the magnitude of benefit for monotherapy olaparib (hazard ratio [HR] = 0.33),4 combination olaparib and bevacizumab (HR = 0.31),6 monotherapy niraparib (HR = 0.4),5 and monotherapy rucaparib (HR = 0.4)10 in terms of progression-free survival is unprecedented. Since front-line treatment is the only opportunity we have for cure, it remains essential, in this author’s opinion, to offer PARP inhibitor maintenance to all patients with BRCA-associated ovarian cancer following a response to front-line platinum-based chemotherapy. There is no role for sequencing PARP inhibitors in later-line therapy here.
Overall survival data from these front-line studies are eagerly awaited. Meanwhile, an indicator of possible benefit is the fact that at 60 months, 48% of patients randomly assigned to olaparib remain free of disease and recurrence as compared with 21% of those patients randomly assigned to placebo.11 So, although overall survival may be similar due to crossover at the time of disease progression, disease-free survival (and possibly cure?) may be very different. We will see.
Cautionary Signals
Further, some cautionary signals arise from the long-term follow-up of patients who received a PARP inhibitor in the recurrent disease setting, when treatment is often continued indefinitely to toxicity or disease progression. Frenel et al presented exploratory data from SOLO-2 indicating that among patients who had recurrence and received another line of platinum, the benefit from platinum was compromised in those who had received olaparib as maintenance therapy, with median progression-free survivals of 7 vs 14 months.12 This is an unbalanced and exploratory analysis but does seem plausible.
What does continuous exposure to a PARP inhibitor cause in terms of acquired resistance mechanisms, and how does this impact subsequent lines of therapy? Outside of the front-line setting, PARP inhibitor use is continued indefinitely, and this may negatively impact therapy after disease progression. However, even with the somewhat worrisome data from Frenel et al, overall survival findings in SOLO-2, although not statistically significant, suggested at least no detriment with the use of maintenance olaparib, with reported medians of 51.7 vs 38.8 months (HR = 0.74, 95% confidence interval [CI] = 0.54–1.0, P = .0537).13 Overall survival data from SOLO-3 are reassuring in this regard as well, with medians of 34.9 and 32.9 months for olaparib and nonplatinum therapy, respectively.14

These two studies were conducted entirely in BRCA-mutated populations. Recently, a Dear Health Care Provider letter released overall survival data from NOVA, suggesting a detriment in certain subgroups. For BRCA-associated cancers, the median overall survival was 43.6 vs 41.6 months for niraparib vs placebo. However, in the BRCA wild-type and especially the BRCA wild-type/homologous recombination deficient (HRD) cohorts, the survival curves cross. For all patients with BRCA wild-type disease, median overall survival was 31.1 vs 36.5 months (HR = 1.10, 95% CI = 0.83–1.46), and for the HRD cohort specifically (excluding BRCA status), median overall survival was 37.3 vs 41.4 months (HR = 1.32, 95% CI = 0.84–2.06).15
Additional Considerations
Several things should be emphasized here. First, overall survival was not a primary endpoint for NOVA, and there are missing data, which complicate the interpretation of these nonanalytic endpoints. Similarly, for SOLO-3, 11% of olaparib recipients and 25% of chemotherapy recipients discontinued study participation before death; so, there is uncertainty in the estimates presented previously as well.14 Second, overall survival data to date from many randomized phase III trials are incomplete; continued reporting of overall survival from completed studies is imperative, as it may further inform how we should sequence PARP inhibitors and in whom.
Finally, Kristeleit et al add to the literature on the baseline prevalence of BRCA reversion mutations in heavily pretreated tumors (6.5% overall). Analysis from SOLO-3 reported a prevalence of 3.5%. These mutations are related to prior platinum exposures and, although rare, render tumors nonresponsive to PARP inhibitors (and likely further platinum). Also related to prior exposures are treatment-related myeloid neoplasms, which occurred in 2% of ARIEL4 patients (all on the rucaparib arm), consistent with SOLO-3 at 2.2% and NRG-GY004 at less than 1% (in less heavily pretreated patients).
In Conclusion
Kristeleit and colleagues are to be congratulated on this contribution to the literature. It is important in that it provides further randomized data for PARP inhibitors as compared with chemotherapy from efficacy and safety standpoints and points to pretreatment evaluation for reversion mutations as “a must do” if using PARP inhibitors in the recurrent setting.
In the time during which this study accrued, the standard of care has dramatically changed. For BRCA-associated cancers, the use of front-line PARP inhibitor maintenance for a time-limited interval (2–3 years) should be considered the standard of care to optimize the chance of potential cure or—if not cure—to achieve a 60% to 70% reduction in the hazard of disease progression or death and reduce the risk of reversion mutations and incident treatment-related myeloid neoplasms.
DISCLOSURE: Dr. Moore reported no conflicts of interest.
REFERENCES
1. Kristeleit R, Lisyanskaya A, Fedenko A, et al: Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): An international, open-label, randomised, phase 3 trial. Lancet Oncol 23:465-478, 2022.
2. Liu JF, Brady MF, Matulonis UA, et al: Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): A randomized, open-label, phase III Trial. J Clin Oncol. March 15, 2022 (early release online).
3. Penson RT, Valencia RV, Cibula D, et al: Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): A randomized phase III Trial. J Clin Oncol 38:1164-1174, 2020.
4. Moore K, Colombo N, Scambia G, et al: Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 379:2495-2505, 2018.
5. González-Martín A, Pothuri B, Vergote I, et al: Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med 381:2391-2402, 2019.
6. Ray-Coquard I, Pautier P, Pignata S, et al: Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med 381:2416-2428, 2019.
7. Kaufman B, Shapira-Frommer R, Schmutzler RK, et al: Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33:244-250, 2015.
8. Swisher EM, Kwan TT, Oza AM, et al: Molecular and clinical determinants of response and resistance to rucaparib for recurrent ovarian cancer treatment in ARIEL2 (parts 1 and 2). Nat Commun 12:2487, 2021.
9. Moore KN, Secord AA, Geller MA, et al: Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): A multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 20:636-648, 2019. Erratum in Lancet Oncol 20:e242, 2019.
10. GOG Foundation: GOG 3020 (ATHENA)/ENGOT-ov45 trial (ATHENA-MONO). Press release. Available at www.gog.org/news/gog-3020-athena-engot-ov45-trial-athena-mono-press-release. Accessed June 3, 2022.
11. Banerjee S, Moore KN, Colombo N, et al: Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 22:1721-1731, 2021.
12. Frenel J, Kim J, Berton-Rigaud DB, et al: Efficacy of subsequent chemotherapy for patients with BRCA1/2 mutated platinum sensitive recurrent epithelial ovarian cancer progressing on olaparib vs placebo: The SOLO2/ENGOT-Ov-21 trial. Ann Oncol 31(suppl 4):S551-S589, 2020.
13. Poveda A, Floquet A, Ledermann JA, et al: Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 22:620-631, 2021.
14. Penson RT, Valencia RV, Colombo N, et al: Final overall survival results from SOLO3: Phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1 and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer. 2022 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer. Abstract 26. Presented March 18, 2022.
15. GlaxoSmithKline: Zejula (Niraparib) Important Drug Warning for the Maintenance Treatment in Recurrent Ovarian Cancer (2L+). Health Care Provider Letter. May 2022.
Dr. Moore is Director of the Oklahoma Tobacco Settlement Endowment Trust Phase I Program and Associate Professor, Section of Gynecologic Oncology, The University of Oklahoma College of Medicine, Oklahoma City.

